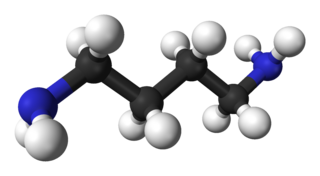
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.
The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer.

A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create two identical DNA duplexes from a single original DNA duplex. During this process, DNA polymerase "reads" the existing DNA strands to create two new strands that match the existing ones. These enzymes catalyze the chemical reaction
Agmatine, also known as 4-aminobutyl-guanidine, was discovered in 1910 by Albrecht Kossel. It is a chemical substance which is naturally created from the amino acid arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis and polyamine metabolism and this provides bases for further research into potential applications.
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. It functions as an intracellular free radical scavenger to protect DNA from free radical attack. Spermine is the chemical primarily responsible for the characteristic odor of semen.

T7 RNA Polymerase is an RNA polymerase from the T7 bacteriophage that catalyzes the formation of RNA from DNA in the 5'→ 3' direction.

Polynucleotide Phosphorylase (PNPase) is a bifunctional enzyme with a phosphorolytic 3' to 5' exoribonuclease activity and a 3'-terminal oligonucleotide polymerase activity. That is, it dismantles the RNA chain starting at the 3' end and working toward the 5' end. It also synthesizes long, highly heteropolymeric tails in vivo. It accounts for all of the observed residual polyadenylation in strains of Escherichia coli missing the normal polyadenylation enzyme. Discovered by Marianne Grunberg-Manago working in Severo Ochoa's lab in 1955, the RNA-polymerization activity of PNPase was initially believed to be responsible for DNA-dependent synthesis of messenger RNA, a notion that was disproven by the late 1950s.
Spermine synthase is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction

Endothelial NOS (eNOS), also known as nitric oxide synthase 3 (NOS3) or constitutive NOS (cNOS), is an enzyme that in humans is encoded by the NOS3 gene located in the 7q35-7q36 region of chromosome 7. This enzyme is one of three isoforms that synthesize nitric oxide (NO), a small gaseous and lipophilic molecule that participates in several biological processes. The other isoforms include neuronal nitric oxide synthase (nNOS), which is constitutively expressed in specific neurons of the brain and inducible nitric oxide synthase (iNOS), whose expression is typically induced in inflammatory diseases. eNOS is primarily responsible for the generation of NO in the vascular endothelium, a monolayer of flat cells lining the interior surface of blood vessels, at the interface between circulating blood in the lumen and the remainder of the vessel wall. NO produced by eNOS in the vascular endothelium plays crucial roles in regulating vascular tone, cellular proliferation, leukocyte adhesion, and platelet aggregation. Therefore, a functional eNOS is essential for a healthy cardiovascular system.

Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the EIF5A gene.

Cyclooxygenase 1 (COX-1), also known as prostaglandin-endoperoxide synthase 1, is an enzyme that in humans is encoded by the PTGS1 gene. In humans it is one of two cyclooxygenases.

Diamine acetyltransferase 1 is an enzyme that in humans is encoded by the SAT1 gene found on the X chromosome.
Activated RNA polymerase II transcriptional coactivator p15 also known as positive cofactor 4 (PC4) or SUB1 homolog is a protein that in humans is encoded by the SUB1 gene. The human SUB1 gene is named after an orthologous gene in yeast.

Spermine oxidase is an enzyme that in humans is encoded by the SMOX gene.

S-Adenosylmethioninamine is a substrate that is required for the biosynthesis of polyamines including spermidine, spermine, and thermospermine. It is produced by decarboxylation of S-adenosyl methionine.
Jerard Hurwitz was an American biochemist who co-discovered RNA polymerase in 1960 along with Sam Weiss, Audrey Stevens, and James Bonner. He most recently worked at the Sloan-Kettering Institute in New York studying DNA replication in eukaryotes and its control.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
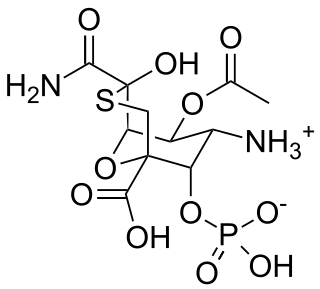
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.

DNA polymerase alpha catalytic subunit is an enzyme that in humans is encoded by the POLA1 gene.
Charles Clifton Richardson is an American biochemist and professor at Harvard University. Richardson received his undergraduate education at Duke University, where he majored in medicine. He received his M.D. at Duke Medical School in 1960. Richardson works as a professor at Harvard Medical School, and he served as editor/associate editor of the Annual Review of Biochemistry from 1972 to 2003. Richardson received the American Chemical Society Award in Biological Chemistry in 1968, as well as numerous other accolades.

















