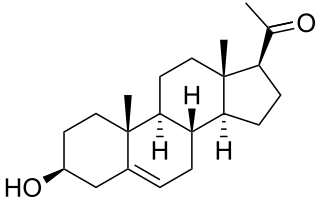
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.

Apolipoprotein B (ApoB) is a protein that in humans is encoded by the APOB gene. It is commonly used to detect risk of atherosclerotic cardiovascular disease.
The steroidogenic acute regulatory protein, commonly referred to as StAR (STARD1), is a transport protein that regulates cholesterol transfer within the mitochondria, which is the rate-limiting step in the production of steroid hormones. It is primarily present in steroid-producing cells, including theca cells and luteal cells in the ovary, Leydig cells in the testis and cell types in the adrenal cortex.

The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane lipoprotein receptor of the low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable homology with the members of this lineage. Discovered in 1992 by T. Yamamoto, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apolipoprotein E-containing triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the VLDLR gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis.

The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands they were subsequently deorphanized.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the LRP8 gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases.
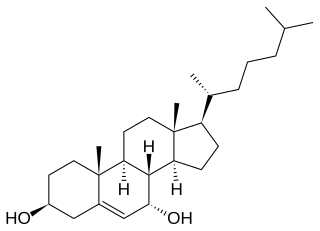
7α-Hydroxycholesterol is a precursor of bile acids, created by cholesterol 7α-hydroxylase (CYP7A1). Its formation is the rate-determining step in bile acid synthesis.
The RAR-related orphan receptors (RORs) are members of the nuclear receptor family of intracellular transcription factors. There are three forms of ROR, ROR-α, -β, and -γ and each is encoded by a separate gene, RORA, RORB, and RORC respectively. The RORs are somewhat unusual in that they appear to bind as monomers to hormone response elements as opposed to the majority of other nuclear receptors which bind as dimers. They bind to DNA elements called ROR response elements (RORE).

Cholesterol 24-hydroxylase, also commonly known as cholesterol 24S-hydroxylase, cholesterol 24-monooxygenase, CYP46, or CYP46A1, is an enzyme that catalyzes the conversion of cholesterol to 24S-hydroxycholesterol. It is responsible for the majority of cholesterol turnover in the human central nervous system. The systematic name of this enzyme class is cholesterol,NADPH:oxygen oxidoreductase (24-hydroxylating).
Peroxisome proliferator-activated receptor alpha (PPAR-α), also known as NR1C1, is a nuclear receptor protein functioning as a transcription factor that in humans is encoded by the PPARA gene. Together with peroxisome proliferator-activated receptor delta and peroxisome proliferator-activated receptor gamma, PPAR-alpha is part of the subfamily of peroxisome proliferator-activated receptors. It was the first member of the PPAR family to be cloned in 1990 by Stephen Green and has been identified as the nuclear receptor for a diverse class of rodent hepatocarcinogens that causes proliferation of peroxisomes.

Liver X receptor alpha (LXR-alpha) is a nuclear receptor protein that in humans is encoded by the NR1H3 gene.

25-hydroxycholesterol 7-alpha-hydroxylase also known as oxysterol and steroid 7-alpha-hydroxylase is an enzyme that in humans is encoded by the CYP7B1 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.

Oxysterol-binding protein 1 is a protein that in humans is encoded by the OSBP gene.

Oxysterol-binding protein-related protein 2 is a protein that in humans is encoded by the OSBPL2 gene.

CYP39A1 also known as oxysterol 7-α-hydroxylase 2 is a protein that in humans is encoded by the CYP39A1 gene.
An oxysterol is a derivative of cholesterol obtained by oxidation involving enzymes and / or pro-oxidants. Such compounds play important roles in various biological processes such as cholesterol homeostasis, lipid metabolism, apoptosis, autophagy, and prenylation of proteins; the mode of action of oxysterols in these effects is still poorly understood. Several oxysterols are associated with age-related diseases such as cardiovascular disease, eye disease, certain neurodegenerative diseases and cancers. The activities of oxysterols in these diseases could be due to their pro-oxidative and pro-inflammatory activities and their ability to act on cellular organelles that can contribute to activate apoptosis and autophagy. There are arguments supporting that oxysterols have important roles in atherosclerosis progression which is involved in several cardiovascular diseases.

27-Hydroxycholesterol (27-HC) is an endogenous oxysterol with multiple biological functions, including activity as a selective estrogen receptor modulator (SERM) and as an agonist of the liver X receptor (LXR). It is a metabolite of cholesterol that is produced by the enzyme CYP27A1.

Soticlestat is an experimental anticonvulsant and cholesterol 24-hydroxylase inhibitor being investigated as a treatment for Dravet syndrome, Lennox–Gastaut syndrome, tuberous sclerosis complex, dup15q syndrome, and CDKL5 deficiency disorder. The development rights to the drug were purchased by Takeda Pharmaceuticals from Ovid Therapeutics in 2021.

















