Deoxyuridine (dU) is a compound and a nucleoside.It belongs to a class of compounds known as Pyrimidine 2'-deoxyribonucleosides and closely resembles the chemical composition of uridine but without the presence of the 2' hydroxyl group. Idoxuridine and Trifluridine are variants of deoxyuridine used as antiviral drugs. They are similar enough to be incorporated as part of DNA replication, but they possess side groups on the uracil component (an iodine and a CF3 group, respectively), that prevent base pairing. A known use of dU is as a precursor in the synthesis of Edoxudine.

Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.

Dehydroandrosterone (DHA), or 5-dehydroandrosterone (5-DHA), also known as isoandrostenolone, as well as androst-5-en-3α-ol-17-one, is an endogenous androgen steroid hormone. It is the 3α-epimer of dehydroepiandrosterone and the 5(6)-dehydrogenated and non-5α-reduced analogue of androsterone (5α-androstan-3α-ol-17-one). DHA is produced in and secreted from the adrenal glands, along with other weak androgens like DHEA, androstenediol, and androstenedione.

UDP-glucuronosyltransferase 2B17 is an enzyme that in humans is encoded by the UGT2B17 gene.

3-Hydroxyisobutyric acid is an intermediate in the metabolism of valine. It is a chiral compound having two enantiomers, D-3-hydroxyisobutyric acid and L-3-hydroxyisobutyric acid.

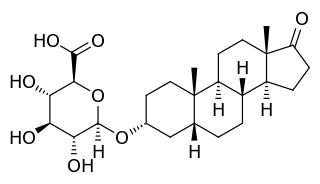
3α-Androstanediol glucuronide (3α-ADG) is a metabolite formed from human androgens; compounds involved in the development and maintenance of sexual characteristics. It is formed by the glucuronidation of both dihydrotestosterone and testosterone, and has been proposed as means of measuring androgenic activity.

Estriol glucuronide (E3G), or oestriol glucuronide, also known as estriol monoglucuronide, as well as estriol 16α-β-D-glucosiduronic acid, is a natural, steroidal estrogen and the glucuronic acid conjugate of estriol. It occurs in high concentrations in the urine of pregnant women as a reversibly formed metabolite of estriol. Estriol glucuronide is a prodrug of estriol, and was the major component of Progynon and Emmenin, estrogenic products manufactured from the urine of pregnant women that were introduced in the 1920s and 1930s and were the first orally active estrogens. Emmenin was succeeded by Premarin, which is sourced from the urine of pregnant mares and was introduced in 1941. Premarin replaced Emmenin due to the fact that it was easier and less expensive to produce.

3α-Etiocholanediol, or simply etiocholanediol, also known as 3α,5β-androstanediol or as etiocholane-3α,17β-diol, is a naturally occurring etiocholane (5β-androstane) steroid and an endogenous metabolite of testosterone. It is formed from 5β-dihydrotestosterone and is further transformed into etiocholanolone.

Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol. It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estradiol. Glucuronides are the most abundant estrogen conjugates.
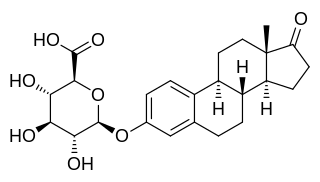
Estrone glucuronide, or estrone-3-D-glucuronide, is a conjugated metabolite of estrone. It is formed from estrone in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estrone. Glucuronides are the most abundant estrogen conjugates and estrone glucuronide is the dominant metabolite of estradiol.

Estriol sulfate glucuronide, or estriol 3-sulfate 16α-glucuronide, is an endogenous, naturally occurring diconjugated metabolite of estriol. It is generated in the liver from estriol sulfate by UDP-glucuronyltransferase and is eventually excreted in the urine by the kidneys. It occurs in high concentrations during pregnancy along with estriol sulfate and estriol glucuronide, and was a component of the early pharmaceutical estrogens Progynon and Emmenin.
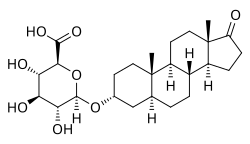
Etiocholanolone glucuronide (ETIO-G) is an endogenous, naturally occurring metabolite of testosterone. It is formed in the liver from etiocholanolone by UDP-glucuronyltransferases. ETIO-G has much higher water solubility than etiocholanolone and is eventually excreted in the urine via the kidneys. Along with androsterone glucuronide, it is one of the major inactive metabolites of testosterone.

Testosterone glucuronide is an endogenous, naturally occurring steroid and minor urinary metabolite of testosterone.

Testosterone sulfate is an endogenous, naturally occurring steroid and minor urinary metabolite of testosterone.

2-Methoxyestrone (2-ME1) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estrone that is formed by catechol O-methyltransferase via the intermediate 2-hydroxyestrone. Unlike estrone but similarly to 2-hydroxyestrone and 2-methoxyestradiol, 2-methoxyestrone has very low affinity for the estrogen receptor and lacks significant estrogenic activity.
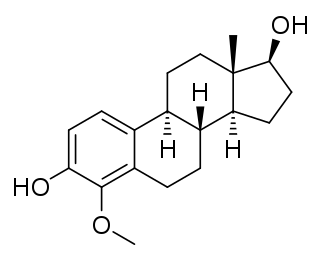
4-Methoxyestradiol (4-ME2) is an endogenous, naturally occurring methoxylated catechol estrogen and metabolite of estradiol that is formed by catechol O-methyltransferase via the intermediate 4-hydroxyestradiol. It has estrogenic activity similarly to estrone and 4-hydroxyestrone.

Estriol 3-glucuronide, or oestriol 3-glucuronide, also known as estriol 3-β-D-glucosiduronic acid, is a natural, steroidal estrogen and a glucuronic acid conjugate of estriol. It is found in the urine of women as a reversibly formed metabolite of estriol. The positional isomer of estriol 3-glucuronide, estriol 16α-glucuronide, also occurs as an endogenous metabolite of estriol, but to a much greater extent in comparison.

Androsterone sulfate, also known as 3α-hydroxy-5α-androstan-17-one 3α-sulfate, is an endogenous, naturally occurring steroid and one of the major urinary metabolites of androgens. It is a steroid sulfate which is formed from sulfation of androsterone by the steroid sulfotransferase SULT2A1 and can be desulfated back into androsterone by steroid sulfatase.

Etiocholanedione, also known as 5β-androstanedione or as etiocholane-3,17-dione, is a naturally occurring etiocholane (5β-androstane) steroid and an endogenous metabolite of androgens like testosterone, dihydrotestosterone, dehydroepiandrosterone (DHEA), and androstenedione. It is the C5 epimer of androstanedione (5α-androstanedione). Although devoid of androgenic activity like other 5β-reduced steroids, etiocholanedione has some biological activity of its own. The compound has been found to possess potent haematopoietic effects in a variety of models. In addition, it has been found to promote weight loss in animals and in a double-blind, placebo-controlled clinical study in humans conducted in 1993. These effects are said to be similar to those of DHEA. Unlike DHEA however, etiocholanedione cannot be metabolized further into steroid hormones like androgens and estrogens.

Estradiol 3-glucuronide (E2-3G), also known as 17β-estradiol 3-(β-D-glucuronide), is a naturally occurring and endogenous estrogen conjugate. It is specifically the C3 glucuronide conjugate of estradiol, the major estrogen in the body. It is formed from estradiol in the liver by UDP-glucuronosyltransferase via attachment of glucuronic acid and is eventually excreted in urine and bile. Similarly to estrogen sulfates like estrone sulfate, estrogen glucuronides have much higher water solubility than do unconjugated estrogens like estradiol.