Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.
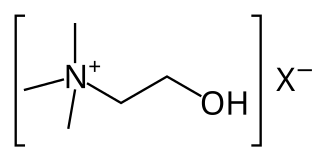
Cholinergic agents are compounds which mimic the action of acetylcholine and/or butyrylcholine. In general, the word "choline" describes the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation. Found in most animal tissues, choline is a primary component of the neurotransmitter acetylcholine and functions with inositol as a basic constituent of lecithin. Choline also prevents fat deposits in the liver and facilitates the movement of fats into cells.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Donepezil, sold under the brand name Aricept among others, is a medication used to treat dementia of the Alzheimer's type. It appears to result in a small benefit in mental function and ability to function. Use, however, has not been shown to change the progression of the disease. Treatment should be stopped if no benefit is seen. It is taken by mouth or via a transdermal patch.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, Sjögren syndrome and underactive bladder.

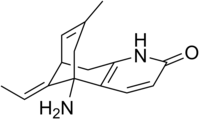
Physostigmine is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and the fruit of the Manchineel tree.
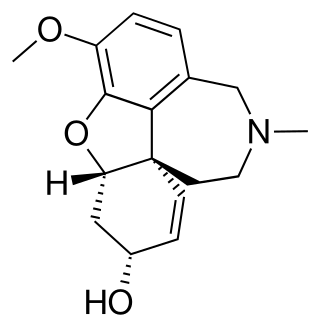
Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments. It is an alkaloid extracted from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
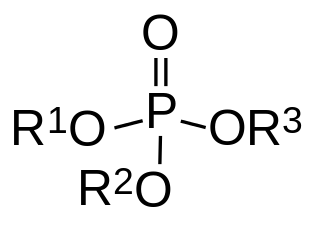
Organophosphate poisoning is poisoning due to organophosphates (OPs). Organophosphates are used as insecticides, medications, and nerve agents. Symptoms include increased saliva and tear production, diarrhea, vomiting, small pupils, sweating, muscle tremors, and confusion. While onset of symptoms is often within minutes to hours, some symptoms can take weeks to appear. Symptoms can last for days to weeks.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:
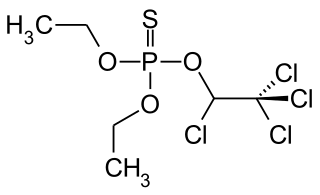
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs).

Rivastigmine is a cholinesterase inhibitor used for the treatment of mild to moderate Alzheimer's disease. The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.

A cholinergic neuron is a nerve cell which mainly uses the neurotransmitter acetylcholine (ACh) to send its messages. Many neurological systems are cholinergic. Cholinergic neurons provide the primary source of acetylcholine to the cerebral cortex, and promote cortical activation during both wakefulness and rapid eye movement sleep. The cholinergic system of neurons has been a main focus of research in aging and neural degradation, specifically as it relates to Alzheimer's disease. The dysfunction and loss of basal forebrain cholinergic neurons and their cortical projections are among the earliest pathological events in Alzheimer's disease.
Carbamate poisoning is poisoning due to exposure to carbamates, which are commonly sold as pesticides around the world. In most respects, it is similar to organophosphate poisoning, though typically less severe or requiring a larger amount of the chemical before symptoms appear.

Phenserine is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.
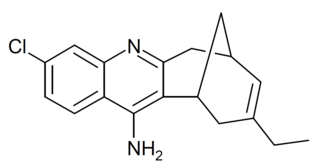
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM, as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors. In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease, and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.