
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, assists in suppressing halitosis, and delivers active ingredients to help prevent tooth decay and gum disease (gingivitis). Owing to differences in composition and fluoride content, not all toothpastes are equally effective in maintaining oral health. The decline of tooth decay during the 20th century has been attributed to the introduction and regular use of fluoride-containing toothpastes worldwide. Large amounts of swallowed toothpaste can be toxic.
Fluoride is an inorganic, monatomic anion of fluorine, with the chemical formula F−
, whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin.

Ornithine transcarbamylase (OTC) is an enzyme that catalyzes the reaction between carbamoyl phosphate (CP) and ornithine (Orn) to form citrulline (Cit) and phosphate (Pi). There are two classes of OTC: anabolic and catabolic. This article focuses on anabolic OTC. Anabolic OTC facilitates the sixth step in the biosynthesis of the amino acid arginine in prokaryotes. In contrast, mammalian OTC plays an essential role in the urea cycle, the purpose of which is to capture toxic ammonia and transform it into urea, a less toxic nitrogen source, for excretion.

Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals, it is an intermediary metabolite in nitrogen disposal through the urea cycle and the synthesis of pyrimidines. Its enzymatic counterpart, carbamoyl phosphate synthetase I, interacts with a class of molecules called sirtuins, NAD dependent protein deacetylases, and ATP to form carbamoyl phosphate. CP then enters the urea cycle in which it reacts with ornithine to form citrulline.
The agents in this list have been classified in Group 2A by the International Agency for Research on Cancer (IARC). The term "agent" encompasses both substances and exposure circumstances that pose a risk. This designation is applied when there is limited evidence of carcinogenicity in humans as well as sufficient evidence of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is inadequate evidence of carcinogenicity in humans along with sufficient evidence of carcinogenicity in experimental animals and strong evidence that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of limited evidence of carcinogenicity in humans.
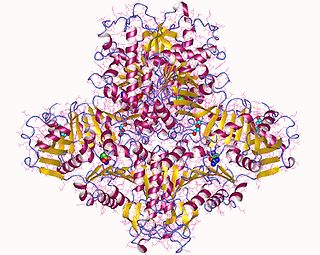
N-Acetylglutamate synthase (NAGS) is an enzyme that catalyses the production of N-acetylglutamate (NAG) from glutamate and acetyl-CoA.
Sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Sulfuryl chloride fluoride is the chemical compound with the formula SO2ClF. It is a colorless, easily condensed gas. It is a tetrahedral molecule.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields an equal amount of ammonium carbamate. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.
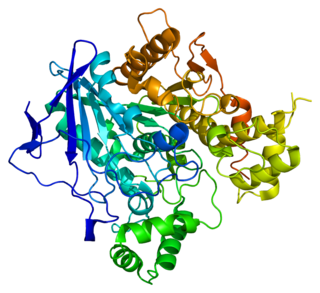
Butyrylcholinesterase, also known as BChE, BuChE, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the BCHE gene.

Carbamoyl phosphate synthetase catalyzes the ATP-dependent synthesis of carbamoyl phosphate from glutamine or ammonia and bicarbonate. This enzyme catalyzes the reaction of ATP and bicarbonate to produce carboxy phosphate and ADP. Carboxy phosphate reacts with ammonia to give carbamic acid. In turn, carbamic acid reacts with a second ATP to give carbamoyl phosphate plus ADP.

Chloride channel protein 2 is a protein that in humans is encoded by the CLCN2 gene. Mutations of this gene have been found to cause leukoencephalopathy and Idiopathic generalised epilepsy, although the latter claim has been disputed. CLCN2 contains a transmembrane region that is involved in chloride ion transport as well two intracellular copies of the CBS domain.
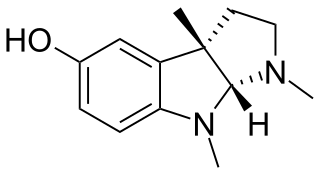
Eseroline is a drug which acts as an opioid agonist. It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible, and it produces fairly potent analgesic effects mediated through the μ-opioid receptor. This mixture of activities gives eseroline an unusual pharmacological profile, although its uses are limited by side effects such as respiratory depression and neurotoxicity.

Dextrallorphan (DXA) is an opioid derivative chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
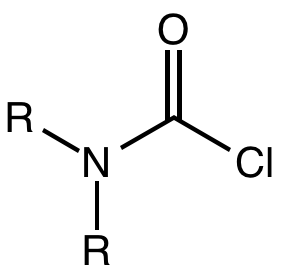
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.
A chloride channel blocker is a type of drug which inhibits the transmission of ions (Cl−) through chloride channels.
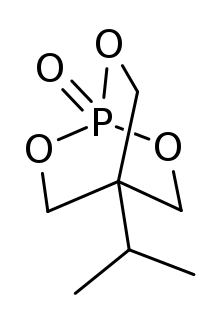
IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.

Schradan, named after Gerhard Schrader, is an obsolete organophosphate insecticide. Schradan itself is a weak cholinesterase inhibitor and requires metabolic activation to become active.
Carbamoyl Phosphate synthetase III is one of the three isoforms of the Carbamoyl Phosphate Synthetase, en enzyme that catalyzes the active production of carbamoyl phosphate in many organisms.













