Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for human and non-human animals; the state of anesthesia they induce is referred to as dissociative anesthesia.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.

In enzymology, a kynurenine 3-monooxygenase (EC 1.14.13.9) is an enzyme that catalyzes the chemical reaction

α-Cobratoxin is a substance of the venom of certain Naja cobras. It is a nicotinic acetylcholine receptor (nAChR) antagonist which causes paralysis by preventing the binding of acetylcholine to the nAChR.
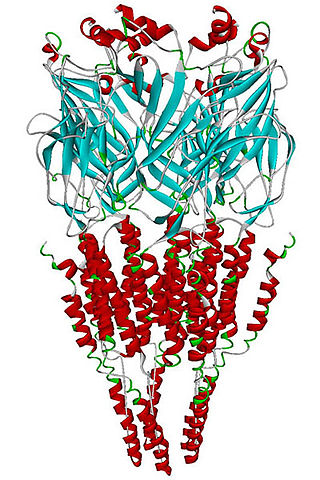
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].

Xanthurenic acid, or xanthurenate, is a metabolic intermediate that accumulates and is excreted by pyridoxine (vitamin B6) deficient animals after the ingestion of tryptophan.

Quinolinic acid, also known as pyridine-2,3-dicarboxylic acid, is a dicarboxylic acid with a pyridine backbone. It is a colorless solid. It is the biosynthetic precursor to niacin.
The glutamate hypothesis of schizophrenia models the subset of pathologic mechanisms of schizophrenia linked to glutamatergic signaling. The hypothesis was initially based on a set of clinical, neuropathological, and, later, genetic findings pointing at a hypofunction of glutamatergic signaling via NMDA receptors. While thought to be more proximal to the root causes of schizophrenia, it does not negate the dopamine hypothesis, and the two may be ultimately brought together by circuit-based models. The development of the hypothesis allowed for the integration of the GABAergic and oscillatory abnormalities into the converging disease model and made it possible to discover the causes of some disruptions.
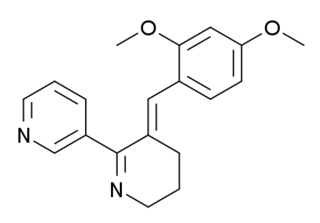
GTS-21 is a drug that has been shown to enhance memory and cognitive function. It has been studied for its potential therapeutic uses, particularly in the treatment of neurodegenerative diseases and psychiatric disorders.

Hypertryptophanemia is a rare autosomal recessive metabolic disorder that results in a massive buildup of the amino acid tryptophan in the blood, with associated symptoms and tryptophanuria.
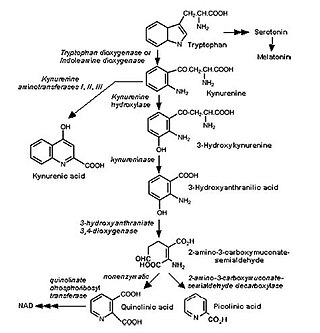
The kynurenine pathway is a metabolic pathway leading to the production of nicotinamide adenine dinucleotide (NAD+). Metabolites involved in the kynurenine pathway include tryptophan, kynurenine, kynurenic acid, xanthurenic acid, quinolinic acid, and 3-hydroxykynurenine. The kynurenine pathway is responsible for total catabolization of tryptophan about 95%. Disruption in the pathway is associated with certain genetic and psychiatric disorders.

PHA-543,613 is a drug that acts as a potent and selective agonist for the α7 subtype of neural nicotinic acetylcholine receptors, with a high level of brain penetration and good oral bioavailability. It is under development as a possible treatment for cognitive deficits in schizophrenia. It reduces excitotoxicity and protects striatal dopaminergic neurons in rat models. It also potentiates cognitive enhancement from memantine, decreases dynorphin release and inhibits GSK-B3.
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.

BNC210 is an anxiolytic drug that acts via negative allosteric modulation of the α7-nicotinic acetylcholine receptor, by Bionomics Limited. It is currently being investigated for the treatment of post traumatic stress disorder. The drug has demonstrated clinically significant anxiety reduction in both animal models and in Phase I trials.
The alpha-3 beta-2 nicotinic receptor, also known as the α3β2 receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β2 subunits.

Hydroxynorketamine (HNK), or 6-hydroxynorketamine, is a minor metabolite of the anesthetic, dissociative, and antidepressant drug ketamine. It is formed by hydroxylation of the intermediate norketamine, another metabolite of ketamine. As of late 2019, (2R,6R)-HNK is in clinical trials for the treatment of depression.