Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals as a neurotransmitter—a chemical message released by nerve cells to send signals to other cells, such as neurons, muscle cells and gland cells. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively.

An acetylcholine receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.
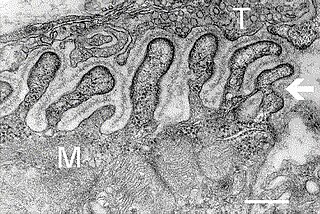
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.

Curare is a common name for various plant extract alkaloid arrow poisons originating from indigenous peoples in Central and South America. Used as a paralyzing agent for hunting and for therapeutic purposes, Curare only becomes active by a direct wound contamination by a poison dart or arrow or via injection. These poisons function by competitively and reversibly inhibiting the nicotinic acetylcholine receptor (nAChR), which is a subtype of acetylcholine receptor found at the neuromuscular junction. This causes weakness of the skeletal muscles and, when administered in a sufficient dose, eventual death by asphyxiation due to paralysis of the diaphragm. Curare is prepared by boiling the bark of one of the dozens of plant alkaloid sources, leaving a dark, heavy paste that can be applied to arrow or dart heads. Historically, curare has been used as an effective treatment for tetanus or strychnine poisoning and as a paralyzing agent for surgical procedures.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

α-Bungarotoxin (α-BTX) is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.

Anatoxin-a, also known as Very Fast Death Factor (VFDF), is a secondary, bicyclic amine alkaloid and cyanotoxin with acute neurotoxicity. It was first discovered in the early 1960s in Canada, and was isolated in 1972. The toxin is produced by multiple genera of cyanobacteria and has been reported in North America, South America, Central America, Europe, Africa, Asia, and Oceania. Symptoms of anatoxin-a toxicity include loss of coordination, muscular fasciculations, convulsions and death by respiratory paralysis. Its mode of action is through the nicotinic acetylcholine receptor (nAchR) where it mimics the binding of the receptor's natural ligand, acetylcholine. As such, anatoxin-a has been used for medicinal purposes to investigate diseases characterized by low acetylcholine levels. Due to its high toxicity and potential presence in drinking water, anatoxin-a poses a threat to animals, including humans. While methods for detection and water treatment exist, scientists have called for more research to improve reliability and efficacy. Anatoxin-a is not to be confused with guanitoxin, another potent cyanotoxin that has a similar mechanism of action to that of anatoxin-a and is produced by many of the same cyanobacteria genera, but is structurally unrelated.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.

α-Cobratoxin is a substance of the venom of certain Naja cobras. It is a nicotinic acetylcholine receptor (nAChR) antagonist which causes paralysis by preventing the binding of acetylcholine to the nAChR.
The alpha-4 beta-2 nicotinic receptor, also known as the α4β2 receptor, is a type of nicotinic acetylcholine receptor implicated in learning, consisting of α4 and β2 subunits. It is located in the brain, where activation yields post- and presynaptic excitation, mainly by increased Na+ and K+ permeability.
Philanthotoxins are components of the venom of the Egyptian solitary wasp Philanthus triangulum, commonly known as the European beewolf. Philanthotoxins are polyamine toxins, a group of toxins isolated from the venom of wasps and spiders which immediately but reversibly paralyze their prey.. δ-philanthotoxin, also known as PhTX-433, is the most active philanthotoxin that can be refined from the venom. PhTX-433 functions by non-selectively blocking excitatory neurotransmitter ion channels, including nicotinic acetylcholine receptors (nAChRs) and ionotropic glutamate receptors (iGluRs). Synthetic analogues, including PhTX-343 and PhTX-12, have been developed to improve selectivity. While the IC50 values of philanthotoxins varies between analogues and receptor subunit composition, the IC50 value of PhTX-433 at the iGluR AMPA receptor naturally expressed in locust leg muscle is 18 μM and the IC50 value at rat nAChRs is 1 μM.

Surugatoxin (SGTX) is a type of venom found in the mid-gut digestive gland of the Japanese ivory mollusk Babyloniajaponica, a carnivorous gastropod. It functions as a ganglionic blocker of nicotinic acetylcholine receptors (nAChRs). The structurally and functionally related neosurugatoxin, also derived from Babylonia japonica, is an even more potent nAChR antagonist than SGTX.

Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the family Dendrobatidae, notably Oophaga histrionica, which are native to Colombia. It is likely that, as with other poison frog alkaloids, histrionicotoxins are not manufactured by the amphibians, but absorbed from insects in their diet and stored in glands in their skin. They are notably less toxic than other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.

α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.

Phantasmidine is a toxic substance derived from the Ecuadorian poisonous frog Anthony's poison arrow frog, more commonly known as the “phantasmal poison frog”. It is a nicotinic agonist, meaning it binds to nicotinic receptors in the body and mimics the effects of the neurotransmitter acetylcholine. This causes the stimulation of the body's parasympathetic nervous system, which induces many inhibitory behaviors in the body such as decreased heart rate.

N,N-Dimethylphenethylamine (N,N-DMPEA) is a substituted phenethylamine that is used as a flavoring agent. It is an alkaloid that was first isolated from the orchid Eria jarensis. Its aroma is described as "sweet, fishy". It is mainly used in cereal, cheese, dairy products, fish, fruit and meat. It is also being used in pre-workout and bodybuilding supplements with claims of a stimulant effect.

Anagyrine is a teratogenic alkaloid commonly found in many species of Lupinus plants. The toxin can cause crooked calf disease if a cow ingests the plant during certain periods of pregnancy.

Guvacoline is a compound with the chemical formula C7H11NO2. It is a component of Areca nut and a precursor of guvacine. It agonizes musacarinic receptors just like arecoline but unlike arecoline it lacks nicotinic activity.

Nereistoxin is a natural product identified in 1962 as the toxic organic compound N,N-dimethyl-1,2-dithiolan-4-amine. It had first been isolated in 1934 from the marine annelid Lumbriconereis heteropoda and acts by blocking the nicotinic acetylcholine receptor. Researchers at Takeda in Japan investigated it as a possible insecticide. They subsequently developed a number of derivatives that were commercialised, including those with the ISO common names bensultap, cartap, thiocyclam and thiosultap.