
Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Mecamylamine is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug. In the United States, it was voluntarily withdrawn from the market in 2009 but was brought to market in 2013 as Vecamyl and eventually was marketed by Turing Pharmaceuticals.

A muscarinic agonist is an agent that activates the activity of the muscarinic acetylcholine receptor. The muscarinic receptor has different subtypes, labelled M1-M5, allowing for further differentiation.
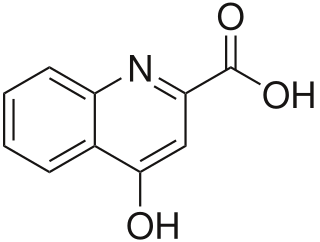
Kynurenic acid is a product of the normal metabolism of amino acid L-tryptophan. It has been shown that kynurenic acid possesses neuroactive activity. It acts as an antiexcitotoxic and anticonvulsant, most likely through acting as an antagonist at excitatory amino acid receptors. Because of this activity, it may influence important neurophysiological and neuropathological processes. As a result, kynurenic acid has been considered for use in therapy in certain neurobiological disorders. Conversely, increased levels of kynurenic acid have also been linked to certain pathological conditions.

Lobeline is a pyridine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.

Neuronal acetylcholine receptor subunit alpha-3, also known as nAChRα3, is a protein that in humans is encoded by the CHRNA3 gene. The protein encoded by this gene is a subunit of certain nicotinic acetylcholine receptors (nAchR). Research with mecamylamine in animals has implicated alpha-3-containing nAChRs in the abusive and addictive properties of ethanol.
The alpha-4 beta-2 nicotinic receptor, also known as the α4β2 receptor, is a type of nicotinic acetylcholine receptor implicated in learning, consisting of α4 and β2 subunits. It is located in the brain, where activation yields post- and presynaptic excitation, mainly by increased Na+ and K+ permeability.

The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].

Cholinergic receptor, nicotinic, alpha 6, also known as nAChRα6, is a protein that in humans is encoded by the CHRNA6 gene. The CHRNA6 gene codes for the α6 nicotinic receptor subunit that is found in certain types of nicotinic acetylcholine receptors found primarily in the brain. Neural nicotinic acetylcholine receptors containing α6 subunits are expressed on dopamine-releasing neurons in the midbrain, and dopamine release following activation of these neurons is thought to be involved in the addictive properties of nicotine. Due to their selective localisation on dopaminergic neurons, α6-containing nACh receptors have also been suggested as a possible therapeutic target for the treatment of Parkinson's disease. In addition to nicotine, research in animals has implicated alpha-6-containing nAChRs in the abusive and addictive properties of ethanol, with mecamylamine demonstrating a potent ability to block these properties.
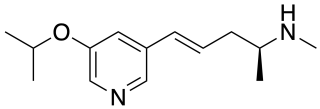
Ispronicline is an experimental drug which acts as a partial agonist at neural nicotinic acetylcholine receptors. It progressed to phase II clinical trials for the treatment of dementia and Alzheimer's disease, but is no longer under development.
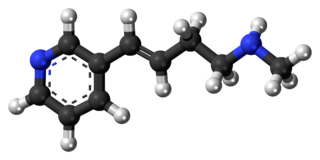
Rivanicline is a drug which acts as a partial agonist at neural nicotinic acetylcholine receptors. It is subtype-selective, binding primarily to the α4β2 subtype. It has nootropic effects and was originally developed as a potential treatment for Alzheimer's disease, but a second action that was subsequently found was that it inhibits the production of Interleukin-8 and thus produces an antiinflammatory effect, and so it has also been developed as a potential treatment for ulcerative colitis. Rivanicline also has stimulant and analgesic actions which are thought to be mediated through stimulation of noradrenaline release, and so it could also have other applications. It has been identified as constituent of tobacco as well.

Tebanicline is a potent synthetic nicotinic (non-opioid) analgesic drug developed by Abbott. It was developed as a less toxic analog of the potent poison dart frog-derived compound epibatidine, which is about 200 times stronger than morphine as an analgesic, but produces extremely dangerous toxic side effects. Like epibatidine, tebanicline showed potent analgesic activity against neuropathic pain in both animal and human trials, but with far less toxicity than its parent compound. It acts as a partial agonist at neuronal nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes.
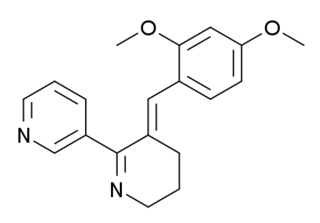
GTS-21 (DMXBA) is a derivative of the natural product anabaseine that acts as a partial agonist at neural nicotinic acetylcholine receptors. It binds to both the α4β2 and α7 subtypes, but activates only the α7 to any significant extent.
A serenic, or antiaggressive agent, is a type of drug which reduces the capacity for irritability and aggression.

PNU-282,987 is a drug that acts as a potent and selective agonist for the α7 subtype of neural nicotinic acetylcholine receptors. In animal studies, it shows nootropic effects, and derivatives may be useful in the treatment of schizophrenia, although PNU-282,987 is not suitable for use in humans because of excessive inhibition of the hERG antitarget. PNU-282987 has been shown to initiate signaling that leads to adult neurogeneis in mammals.

ABT-202 is a drug developed by Abbott, which acts as an agonist at neural nicotinic acetylcholine receptors and has been researched for use as an analgesic, although it has not passed clinical trials.

A-366,833 is a drug developed by Abbott, which acts as an agonist at neural nicotinic acetylcholine receptors selective for the α4β2 subtype, and has been researched for use as an analgesic, although it has not passed clinical trials. Its structure has a nicotinonitrile (3-cyanopyridine) core bound through C5 to the N6 of (1R,5S)-3,6-diazabicyclo[3.2.0]heptane.
TC-1698 is a drug developed by Targacept which acts as a partial agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has neuroprotective effects in animal studies, and has been used as a lead compound to find further potent derivatives.

BP-897 is a drug used in scientific research which acts as a potent selective dopamine D3 receptor partial agonist with an in vitro intrinsic activity of ~0.6 and ~70x greater affinity for D3 over D2 receptors and is suspected to have partial agonist or antagonist activity in vivo. It has mainly been used in the study of treatments for cocaine addiction. A study comparing BP-897 with the potent, antagonistic, and highly D3 selective SB-277,011-A found, "SB 277011-A (1–10 mg/kg) was able to block cue-induced reinstatement of nicotine-seeking, indicating that DRD3 selective antagonism may be an effective approach to prevent relapse for nicotine. In contrast, BP 897 did not block the cue-induced reinstatement of nicotine-seeking or nicotine-taking under the FR5 schedule."

















