An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.

Neurotrophins are a family of proteins that induce the survival, development, and function of neurons.
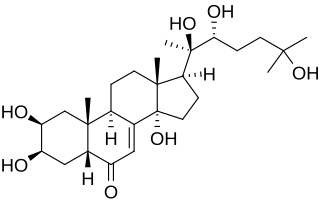
20-Hydroxyecdysone is a naturally occurring ecdysteroid hormone which controls the ecdysis (moulting) and metamorphosis of arthropods. It is therefore one of the most common moulting hormones in insects, crabs, etc. It is also a phytoecdysteroid produced by various plants, including Cyanotis vaga, Ajuga turkestanica and Rhaponticum carthamoides where its purpose is presumably to disrupt the development and reproduction of insect pests. In arthropods, 20-hydroxyecdysone acts through the ecdysone receptor. Although mammals lack this receptor, 20-hydroxyecdysone affects mammalian biological systems. 20-Hydroxyecdysone is an ingredient of some supplements that aim to enhance physical performance. In humans, it is hypothesized to bind to the estrogen receptor beta (ERβ) protein-coding gene.

Phenylpiracetam, is a phenylated analog of the drug piracetam. It was developed in 1983 as a medication for Soviet Cosmonauts to treat the prolonged stresses of working in space. Phenylpiracetam was created at the Russian Academy of Sciences Institute of Biomedical Problems in an effort led by psychopharmacologist Valentina Ivanovna Akhapkina. In Russia it is now available as a prescription drug. Research on animals has indicated that phenylpiracetam may have anti-amnesic, antidepressant, anticonvulsant, anxiolytic, and memory enhancement effects.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.
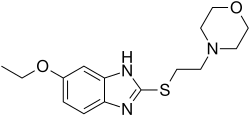
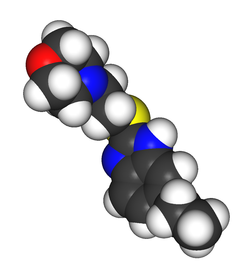
Gidazepam, also known as hydazepam or hidazepam, is a drug which is an atypical benzodiazepine derivative, developed in the Soviet Union. It is a selectively anxiolytic benzodiazepine. It also has therapeutic value in the management of certain cardiovascular disorders.

Diproqualone is a quinazolinone class GABAergic and is an analogue of methaqualone developed in the late 1950s by a team at Nogentaise de Produits Chimique. It was marketed primarily in France and some other European countries. It has sedative, anxiolytic, antihistamine and analgesic properties, resulting from its agonist activity at the β subtype of the GABAa receptor, antagonist activity at all histamine receptors, inhibition of the cyclooxygenase-1 enzyme, and possibly its agonist activity at both the sigma-1 receptor and sigma-2 receptor. Diproqualone is used primarily for the treatment of inflammatory pain associated with osteoarthritis and rheumatoid arthritis, and more rarely for treating insomnia, anxiety and neuralgia.

Latrepirdine is an antihistamine drug which has been used clinically in Russia since 1983.
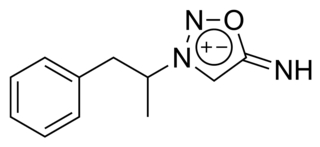
Feprosidnine (Sydnophen) is a stimulant drug which was developed in the USSR in the 1970s. It is structurally related to another Russian drug mesocarb but unlike mesocarb, was withdrawn earlier from production. In comparison with mesocarb it has own antidepressant activity, which makes it useful in treating depressions. Indications of feprosidnine included apathic, asthenic depressions, fatigue, apathic syndrome, narcolepsy and other similar conditions. Therapeutic range of doses: 10-50mg a day. Sydnophen has multiple mechanisms of action, the relative importance of which has not been clearly established. Effects on the body include reversible monoamine oxidase inhibition, cholinergic, adrenergic, opioid and nitric oxide donating actions, all of which may contribute to its pharmacological effects to some extent.

SB-205384 is an anxiolytic drug. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

SB-216641 is a drug which is a selective antagonist for the serotonin receptor 5-HT1B, with around 25x selectivity over the closely related 5-HT1D receptor. It is used in scientific research, and has demonstrated anxiolytic effects in animal studies.

Bromantane, sold under the brand name Ladasten, is an atypical psychostimulant and anxiolytic drug of the adamantane family related to amantadine and memantine which is used in Russia in the treatment of neurasthenia. Although the effects of the bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and it is distinct in its properties relative to typical psychostimulants such as amphetamine. Because of its unique aspects, bromantane has sometimes been described instead as an adaptogen and actoprotector.

N-Phenylacetyl-l-prolylglycine ethyl ester is promoted as a nootropic and is a prodrug of cyclic glycine-proline. Other names include the brand name Noopept, developmental code GVS-111; proposed INN omberacetam.

Selank is a nootropic, anxiolytic peptide based drug developed by the Institute of Molecular Genetics of the Russian Academy of Sciences. Selank is a heptapeptide with the sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro (TKPRPGP). It is a synthetic analogue of human tuftsin.

Emoxypine (2-ethyl-6-methyl-3-hydroxypyridine), also known as Mexidol or Mexifin when used as the succinate salt, is an antioxidant actoprotector manufactured by Pharmasoft Pharmaceuticals. Its chemical structure resembles that of pyridoxine (a type of vitamin B6).

Benzobarbital (Benzonal) is a barbiturate derivative. It has anticonvulsant effects and has been used for the treatment of epilepsy.

Nooglutyl is a nootropic agent that was studied at the Research Institute of Pharmacology, Russian Academy of Medical Sciences as a potential treatment for amnesia.

Flusoxolol is a selective beta-1 receptor blocker.
Neurotrophin mimetics are small molecules or peptide like molecules that can modulate the action of the neurotrophin receptor. One of the main causes of neurodegeneration involves changes in the expression of neurotrophins (NTs) and/or their receptors. Indeed, these imbalances or changes in their activity, lead to neuronal damage resulting in neurological and neurodegenerative conditions. The therapeutic properties of neurotrophins attracted the focus of many researchers during the years, but the poor pharmacokinetic properties, such as reduced bioavailability and low metabolic stability, the hyperalgesia, the inability to penetrate the blood–brain barrier and the short half-lives render the large neurotrophin proteins not suitable to be implemented as drugs.