
Antipsychotics, previously known as neuroleptics and major tranquilizers, are a class of psychotropic medication primarily used to manage psychosis, principally in schizophrenia but also in a range of other psychotic disorders. They are also the mainstay, together with mood stabilizers, in the treatment of bipolar disorder. Moreover, they are also used as adjuncts in the treatment of treatment-resistant major depressive disorder.

Haloperidol, sold under the brand name Haldol among others, is a typical antipsychotic medication. Haloperidol is used in the treatment of schizophrenia, tics in Tourette syndrome, mania in bipolar disorder, delirium, agitation, acute psychosis, and hallucinations from alcohol withdrawal. It may be used by mouth or injection into a muscle or a vein. Haloperidol typically works within 30 to 60 minutes. A long-acting formulation may be used as an injection every four weeks by people with schizophrenia or related illnesses, who either forget or refuse to take the medication by mouth.

Typical antipsychotics are a class of antipsychotic drugs first developed in the 1950s and used to treat psychosis. Typical antipsychotics may also be used for the treatment of acute mania, agitation, and other conditions. The first typical antipsychotics to come into medical use were the phenothiazines, namely chlorpromazine which was discovered serendipitously. Another prominent grouping of antipsychotics are the butyrophenones, an example of which is haloperidol. The newer, second-generation antipsychotics, also known as atypical antipsychotics, have largely supplanted the use of typical antipsychotics as first-line agents due to the higher risk of movement disorders in the latter.

Risperidone, sold under the brand name Risperdal among others, is an atypical antipsychotic used to treat schizophrenia and bipolar disorder. It is taken either by mouth or by injection. The injectable versions are long-acting and last for 2–4 weeks.

Perphenazine is a typical antipsychotic drug. Chemically, it is classified as a piperazinyl phenothiazine. Originally marketed in the United States as Trilafon, it has been in clinical use for decades.
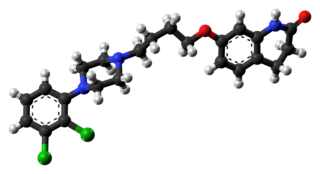
Aripiprazole, sold under the brand names Abilify and Aristada, among others, is an atypical antipsychotic. It is primarily used in the treatment of schizophrenia and bipolar disorder; other uses include as an add-on treatment in major depressive disorder and obsessive compulsive disorder (OCD), tic disorders, and irritability associated with autism. Aripiprazole is taken by mouth or via injection into a muscle. A Cochrane review found low-quality evidence of effectiveness in treating schizophrenia.
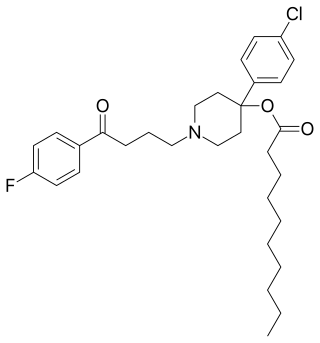
Haloperidol decanoate, sold under the brand name Haldol Decanoate among others, is a typical antipsychotic which is used in the treatment of schizophrenia. It is administered by injection into muscle at a dose of 100 to 200 mg once every 4 weeks or monthly. The dorsogluteal site is recommended. A 3.75-cm (1.5-inch), 21-gauge needle is generally used, but obese individuals may require a 6.5-cm (2.5-inch) needle to ensure that the drug is indeed injected intramuscularly and not subcutaneously. Haloperidol decanoate is provided in the form of 50 or 100 mg/mL oil solution of sesame oil and benzyl alcohol in ampoules or pre-filled syringes. Its elimination half-life after multiple doses is 21 days. The medication is marketed in many countries throughout the world.

Flupentixol (INN), also known as flupenthixol, marketed under brand names such as Depixol and Fluanxol is a typical antipsychotic drug of the thioxanthene class. It was introduced in 1965 by Lundbeck. In addition to single drug preparations, it is also available as flupentixol/melitracen—a combination product containing both melitracen and flupentixol . Flupentixol is not approved for use in the United States. It is, however, approved for use in the UK, Australia, Canada, Russian Federation, South Africa, New Zealand, Philippines, Iran, Germany, and various other countries.

Paliperidone, sold under the brand name Invega among others, is an atypical antipsychotic. It is mainly used to treat schizophrenia and schizoaffective disorder. It is marketed by Janssen Pharmaceuticals.

Fluspirilene is a diphenylbutylpiperidine typical antipsychotic drug, used for the treatment of schizophrenia. It is administered intramuscularly. It was discovered at Janssen Pharmaceutica in 1963. A 2007 systematic review investigated the efficacy of fluspirilene decanoate for people with schizophrenia:
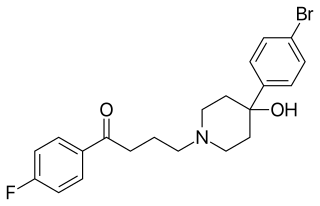
Bromperidol, sold under the brand names Bromidol and Impromen among others, is a typical antipsychotic of the butyrophenone group which is used in the treatment of schizophrenia. It was discovered at Janssen Pharmaceutica in 1966. An ester prodrug, bromperidol decanoate, is a long-acting form of bromperidol used as a depot injectable.

Zuclopenthixol, also known as zuclopentixol, is a medication used to treat schizophrenia and other psychoses. It is classed, pharmacologically, as a typical antipsychotic. Chemically it is a thioxanthene. It is the cis-isomer of clopenthixol. Clopenthixol was introduced in 1961, while zuclopenthixol was introduced in 1978.

Clopenthixol (Sordinol), also known as clopentixol, is a typical antipsychotic drug of the thioxanthene class. It was introduced by Lundbeck in 1961.

Pipotiazine (Piportil), also known as pipothiazine, is a typical antipsychotic of the phenothiazine class used in the United Kingdom and other countries for the treatment of schizophrenia. Its properties are similar to those of chlorpromazine. A 2004 systematic review investigated its efficacy for people with schizophrenia:

Aripiprazole lauroxil, sold under the brand name Aristada, is a long-acting injectable atypical antipsychotic that was developed by Alkermes. It is an N-acyloxymethyl prodrug of aripiprazole that is administered via intramuscular injection once every four to eight weeks for the treatment of schizophrenia. Aripiprazole lauroxil was approved by the U.S. Food and Drug Administration (FDA) on 5 October 2015.
Viscoleo is a thin or low-viscosity vegetable oil. It is specifically a proprietary form of fractionated coconut oil and a medium-chain triglyceride (MCT) oil. It is prepared from the dried, solid endosperm of the fruit Cocos nucifera via hydrolysis, fractionation, and purification. Viscoleo is composed of the medium-chain fatty acids caprylic acid (C8) (55–60%), capric acid (C10) (40%), lauric acid (C12) (1–5%), and caproic acid (C6) (0.5%). It is used as an oil vehicle for several depot antipsychotics including clopentixol decanoate, flupentixol decanoate, pipotiazine palmitate, zuclopentixol acetate, and zuclopentixol decanoate. Injectable antipsychotics using Viscoleo as a carrier may be absorbed more rapidly and have shorter durations than preparations using sesame oil.
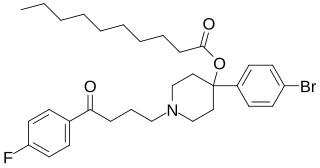
Bromperidol decanoate, sold under the brand names Bromidol Depot, Bromodol Decanoato, and Impromen Decanoas, is an antipsychotic which has been marketed in Europe and Latin America. It is an antipsychotic ester and long-acting prodrug of bromperidol which is administered by depot intramuscular injection once every 4 weeks.
Perphenazine enanthate, sold under the brand name Trilafon Enantat among others, is a typical antipsychotic and a depot antipsychotic ester which is used in the treatment of schizophrenia and has been marketed in Europe. It is formulated in sesame oil and administered by intramuscular injection and acts as a long-lasting prodrug of perphenazine. Perphenazine enanthate is used at a dose of 25 to 200 mg once every 2 weeks by injection, with a time to peak levels of 2 to 3 days and an elimination half-life of 4 to 7 days.

Oxyprothepin decanoate, sold under the brand name Meclopin, is a typical antipsychotic which was used in the treatment of schizophrenia in the Czech Republic but is no longer marketed. It is administered by depot injection into muscle. The medication has an approximate duration of 2 to 3 weeks. The history of oxyprothepin decanoate has been reviewed.

An antipsychotic ester is an ester of an antipsychotic. They are used clinically as prodrugs to increase fat solubility and thereby prolong duration when antipsychotics are used as depot injectables.



















