
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
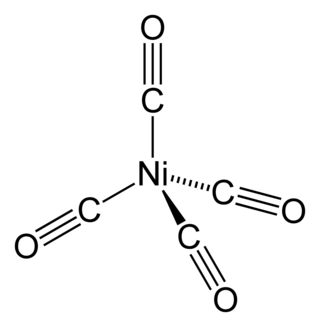
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Triiron dodecarbonyl is the organoiron compound with the formula Fe3(CO)12. It is a dark green solid that sublimes under vacuum. It is soluble in nonpolar organic solvents to give intensely green solutions. Most low-nuclearity clusters are pale yellow or orange. Hot solutions of Fe3(CO)12 decompose to an iron mirror, which can be pyrophoric in air.The solid decomposes slowly in air, and thus samples are typically stored cold under an inert atmosphere. It is a more reactive source of iron(0) than iron pentacarbonyl.
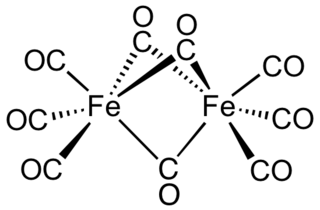
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.
A metallacarboxylic acid is a metal complex with the ligand CO2H. These compounds are intermediates in reactions that involve carbon monoxide and carbon dioxide, these species are intermediates in the water gas shift reaction. Metallacarboxylic acids are also called hydroxycarbonyls.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Metal carbonyl hydrides are complexes of transition metals with carbon monoxide and hydride as ligands. These complexes are useful in organic synthesis as catalysts in homogeneous catalysis, such as hydroformylation.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
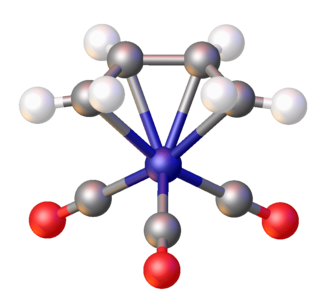
(Butadiene)iron tricarbonyl is an organoiron compound with the formula (C4H6)Fe(CO)3. It is a well-studied metal complex of butadiene. An orange-colored viscous liquid that freezes just below room temperature, the compound adopts a piano stool structure.
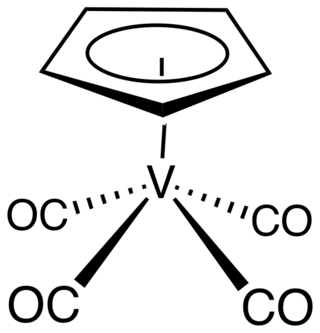
Cyclopentadienylvanadium tetracarbonyl is the organovanadium compound with the formula (C5H5)V(CO)4. An orange, diamagnetic solid, it is the principal cyclopentadienyl carbonyl of vanadium. It can be prepared by heating a solution of vanadocene under high pressure of carbon monoxide. As confirmed by X-ray crystallography, the coordination sphere of vanadium consists of η5-cyclopentadienyl and four carbonyl ligands. The molecule is a four-legged piano stool complex. The compound is soluble in common organic solvents. The compound has no commercial applications.

Tricarbonylbis(triphenylphosphine)iron(0) is a coordination complex with the formula Fe(CO)3(PPh3)2 (Ph = C6H5). A yellow solid, this complex is derived from iron pentacarbonyl by replacement of two carbonyl ligands by triphenylphosphine (PPh3).

(Triphenylphosphine)iron tetracarbonyl is a coordination complex with the formula Fe(CO)4(PPh3) (Ph = C6H5). An off-white solid, this complex is derived from iron pentacarbonyl by replacement of one carbonyl ligand by triphenylphosphine (PPh3).
Potassium tetracarbonyliron hydride is the inorganic salt with the formula K[HFe(CO)4]. A pale yellow solid, it is the potassium salt of [HFe(CO)4]−, which is the conjugate base of iron tetracarbonyl dihydride:
In organometallic chemistry, (diene)iron tricarbonyl describes a diverse family of related coordination complexes consisting of a diene ligand coordinated to a Fe(CO)3 center. Often the diene is conjugated, e.g., butadiene, but the family includes nonconjugated dienes as well. The compounds are yellow, air-stable, often low-melting, and soluble in hydrocarbon solvents. The motif is so robust that even unstable dienes form easily characterized derivatives, such as norbornadienone and cyclobutadiene.






















