Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

Contact lenses, or simply contacts, are thin lenses placed directly on the surface of the eyes. Contact lenses are ocular prosthetic devices used by over 150 million people worldwide, and they can be worn to correct vision or for cosmetic or therapeutic reasons. In 2010, the worldwide market for contact lenses was estimated at $6.1 billion, while the US soft lens market was estimated at $2.1 billion. Multiple analysts estimated that the global market for contact lenses would reach $11.7 billion by 2015. As of 2010, the average age of contact lens wearers globally was 31 years old, and two-thirds of wearers were female.

Calcium carbide, also known as calcium acetylide, is a chemical compound with the chemical formula of CaC2. Its main use industrially is in the production of acetylene and calcium cyanamide.

In chemistry, a phosphide is a compound containing the P3− ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides, which are solids consisting of anionic chains or clusters of phosphorus. Phosphides are known with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, and Po. Finally, some phosphides are molecular.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.
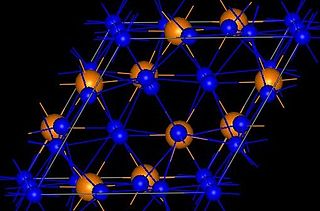
Copper phosphide, Cu3P, also copper(I) phosphide, cuprous phosphide, cuprophosphorus and phosphor copper, is a compound of copper and phosphorus, a phosphide of copper. It has the appearance of yellowish-grey very brittle mass of crystalline structure. It does not react with water.
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Sodium phosphide is the inorganic compound with the formula Na3P. It is a black solid. It is often described as Na+ salt of the P3− anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.
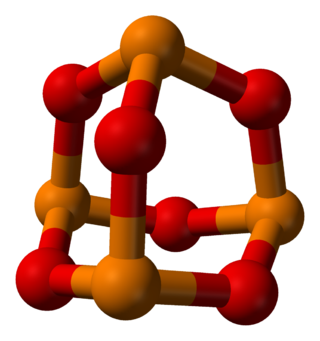
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.

Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO
3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+
and carbonate anions CO2−
3.
Deoxidization is a method used in metallurgy to remove the oxygen content during steel manufacturing. In contrast, antioxidants are used for stabilization, such as in the storage of food. Deoxidation is important in the steelmaking process as oxygen is often detrimental to the quality of steel produced. Deoxidization is mainly achieved by adding a separate chemical species to neutralize the effects of oxygen or by directly removing the oxygen.
Strontium phosphide is an inorganic compound of strontium and phosphorus with the chemical formula Sr
3P
2. The compound looks like black crystalline material.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.

Iron–hydrogen alloy, also known as iron hydride, is an alloy of iron and hydrogen and other elements. Because of its lability when removed from a hydrogen atmosphere, it has no uses as a structural material.
Holmium phosphide is a binary inorganic compound of holmium and phosphorus with the chemical formula HoP. The compound forms dark crystals and does not dissolve in water.
Gadolinium phosphide is an inorganic compound of gadolinium and phosphorus with the chemical formula GdP.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Potassium phosphide is an inorganic semiconductor compound with the formula K3P. It appears as a white crystalline solid or powder. It reacts violently with water and is toxic via ingestion, inhalation and skin absorption. It has a hexagonal structure.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
Platinum diphosphide is a binary inorganic compound of platinum metal and phosphorus with the chemical formula PtP2.










