In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.
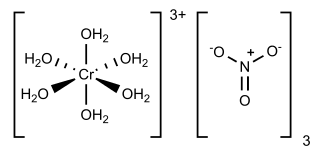
Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hygroscopic solid. An anhydrous green form is also known. Chromium(III) nitrate compounds are of a limited commercial importance, finding some applications in the dyeing industry. It is common in academic laboratories for the synthesis of chromium coordination complexes.

Ferrous oxalate (iron(II) oxalate) is an inorganic compound with the formula FeC2O4 · xH2O where x is typically 2. These are orange compounds, poorly soluble in water.

1,2-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two adjacent oxygen atoms by dicyanomethylene groups =C(−C≡N)2.
This list gives an overview of the classification of non-silicate minerals and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, not IMA approved minerals, not named minerals are mostly excluded. Mostly major groups only, or groupings used by New Dana Classification and Mindat.

Chromium(II) sulfate is an inorganic compound with the chemical formula CrSO4. It often comes as hydrates CrSO4·nH2O. Several hydrated salts are known. The pentahydrate CrSO4·5H2O is a blue solid that dissolves readily in water. Solutions of chromium(II) are easily oxidized by air to Cr(III) species. Solutions of Cr(II) are used as specialized reducing agents of value in organic synthesis.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.

Thulium(III) acetate is the acetate salt of thulium, with the chemical formula of Tm(CH3COO)3. It can exist in the tetrahydrate or the anhydrous form.

Erbium(III) selenate is an inorganic compound, with the chemical formula Er2(SeO4)3. It exists as an anhydrate or an octahydrate.










