
Selenium is a chemical element; it has symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while Se-methylselenocysteine, or its γ-glutamyl derivative, is the major form of selenium found in Astragalus, Allium, and Brassica species. In vivo, selenomethionine is randomly incorporated instead of methionine. Selenomethionine is readily oxidized.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite. Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.
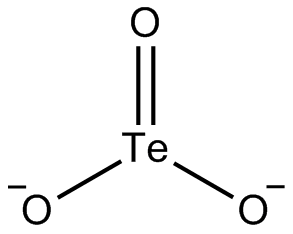
The tellurite ion is TeO2−
3. A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite. A mineral with the formula TeO2 is called tellurite.

Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by O=Se(OH)2. It is the principal oxoacid of selenium; the other being selenic acid.

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.

Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium.
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
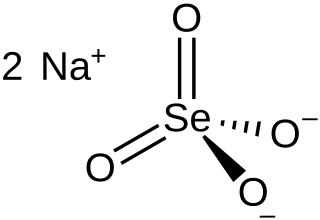
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.

Potassium selenate, K
2SeO
4, is an odorless, white solid that forms as the potassium salt of selenic acid.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.
The nitrate selenites are mixed anion compounds containing distinct nitrate (NO3−)and selenite (SO32−) groups. The compounds are colourless unless coloured by cations.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
A nitrate nitrite, or nitrite nitrate, is a coordination complex or other chemical compound that contains both nitrite and nitrate anions (NO3− and NO2−). They are mixed-anion compounds, and they are mixed-valence compounds. Some have third anions. Many nitrite nitrate compounds are coordination complexes of cobalt. Such a substance was discovered by Wolcott Gibbs and Frederick Genth in 1857.












