
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης, meaning 'violet'.
In chemistry, a halide is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX. Many salts are halides; the hal- syllable in halide and halite reflects this correlation. All Group 1 metals form halides that are white solids at room temperature.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite.
A silver halide is one of the chemical compounds that can form between the element silver (Ag) and one of the halogens. In particular, bromine (Br), chlorine (Cl), iodine (I) and fluorine (F) may each combine with silver to produce silver bromide (AgBr), silver chloride (AgCl), silver iodide (AgI), and four forms of silver fluoride, respectively.

Silver chloride is an inorganic chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite.

In chemistry, triiodide usually refers to the triiodide ion, I−
3. This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+[I3]−) and ammonium triiodide ([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion leaves its place in the structure, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect. In ionic compounds, the vacancy and interstitial defect involved are oppositely charged and one might expect them to be located close to each other due to electrostatic attraction. However, this is not likely the case in real material due to smaller entropy of such a coupled defect, or because the two defects might collapse into each other. Also, because such coupled complex defects are stoichiometric, their concentration will be independent of chemical conditions.
Beta-alumina solid electrolyte (BASE) is a fast ion conductor material used as a membrane in several types of molten salt electrochemical cell. Currently there is no known substitute available. β-Alumina exhibits an unusual layered crystal structure which enables very fast ion transport. β-Alumina is not an isomorphic form of aluminium oxide (Al2O3), but a sodium polyaluminate. It is a hard polycrystalline ceramic, which, when prepared as an electrolyte, is complexed with a mobile ion, such as Na+, K+, Li+, Ag+, H+, Pb2+, Sr2+ or Ba2+ depending on the application. β-Alumina is a good conductor of its mobile ion yet allows no non-ionic (i.e., electronic) conductivity. The crystal structure of the β-alumina provides an essential rigid framework with channels along which the ionic species of the solid can migrate. Ion transport involves hopping from site to site along these channels. Since the 1970's this technology has been thoroughly developed, resulting in interesting applications. Its special characteristics on ion and electrical conductivity make this material extremely interesting in the field of energy storage.

In materials science, fast ion conductors are solid conductors with highly mobile ions. These materials are important in the area of solid state ionics, and are also known as solid electrolytes and superionic conductors. These materials are useful in batteries and various sensors. Fast ion conductors are used primarily in solid oxide fuel cells. As solid electrolytes they allow the movement of ions without the need for a liquid or soft membrane separating the electrodes. The phenomenon relies on the hopping of ions through an otherwise rigid crystal structure.

Silver is a relatively unreactive metal, although it can form several compounds. The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass. Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.

Ionic conductivity is a measure of a substance's tendency towards ionic conduction. Ionic conduction is the movement of ions. The phenomenon is observed in solids and solutions. Ionic conduction is one mechanism of current.
An advanced superionic conductor (AdSIC) in materials science, is fast ion conductor that has a crystal structure close to optimal for fast ion transport (FIT).
Rubidium silver iodide is a ternary inorganic compound with the formula RbAg4I5. Its conductivity involves the movement of silver ions within the crystal lattice. It was discovered while searching for chemicals which had the ionic conductivity properties of alpha-phase silver iodide at temperatures below 146 °C for AgI.
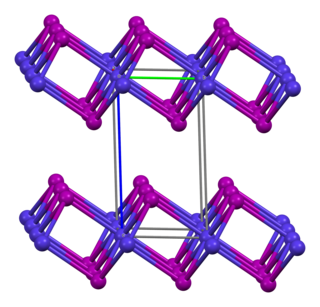
Cobalt(II) iodide or cobaltous iodide are the inorganic compounds with the formula CoI2 and the hexahydrate CoI2(H2O)6. These salts are the principal iodides of cobalt.

Solid-state ionics is the study of ionic-electronic mixed conductor and fully ionic conductors and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites. Solid-state ionic devices, such as solid oxide fuel cells, can be much more reliable and long-lasting, especially under harsh conditions, than comparable devices with fluid electrolytes.

I-III-VI2 semiconductors are solid semiconducting materials that contain three or more chemical elements belonging to groups I, III and VI (IUPAC groups 1/11, 13 and 16) of the periodic table. They usually involve two metals and one chalcogen. Some of these materials have a direct bandgap, Eg, of approximately 1.5 eV, which makes them efficient absorbers of sunlight and thus potential solar cell materials. A fourth element is often added to a I-III-VI2 material to tune the bandgap for maximum solar cell efficiency. A representative example is copper indium gallium selenide (CuInxGa(1–x)Se2, Eg = 1.7–1.0 eV for x = 0–1), which is used in copper indium gallium selenide solar cells.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula Hf I3. It is a black solid.


















