
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C, which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion. The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all commonly available elements and fluorine has the highest electronegativity.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
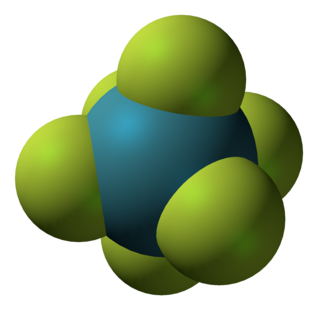
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.

Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application.

Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.

Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless salt is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.
Thallium(I) iodide is a chemical compound with the formula . It is unusual in being one of the few water-insoluble metal iodides, along with , , , , and .
Caesium cadmium chloride (CsCdCl3) is a synthetic crystalline material. It belongs to the AMX3 group (where A=alkali metal, M=bivalent metal, X=halogen ions). It crystallizes in a hexagonal space group P63/mmc with unit cell lengths a = 7.403 Å and c = 18.406 Å, with one cadmium ion having D3d symmetry and the other having C3v symmetry.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Copper hydride is an inorganic compound with the chemical formula CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.

Caesium oxalate, or dicesium oxalate, or cesium oxalate is a chemical compound with the chemical formula Cs2C2O4. It is a caesium salt of oxalic acid. It consists of caesium cations Cs+ and oxalate anions C2O2−4.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.

Phosphonium iodide is a chemical compound with the formula PH
4I. It is an example of a salt containing an unsubstituted phosphonium cation. Phosphonium iodide is commonly used as storage for phosphine and as a reagent for substituting phosphorus into organic molecules.

Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.
Caesium sesquioxide is a chemical compound with the formula Cs2O3 or more accurately Cs4O6. It is an oxide of caesium containing oxygen in different oxidation states. It consists of caesium cations Cs+, superoxide anions O−2 and peroxide anions O2−2. Caesium in this compound has an oxidation state of +1, while oxygen in superoxide has an oxidation state of −1/2 and oxygen in peroxide has an oxidation state of −1. This compound has a structural formula of (Cs+)4(O−2)2(O2−2). Compared to the other caesium oxides, this phase is less well studied, but has been long present in the literature. It can be created by thermal decomposition of caesium superoxide at 290 °C.

Caesium superoxide is a chemical compound with the chemical formula CsO2. It consists of caesium cations Cs+ and superoxide anions O−2. It is an orange solid.



















