
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.

Rubidium is a chemical element; it has symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Terbium is a chemical element; it has symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.

Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.

Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
In chemistry, a reactivity series (or activity series) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. It is used to summarize information about the reactions of metals with acids and water, single displacement reactions and the extraction of metals from their ores.

Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.
Carbothermic reactions involve the reduction of substances, often metal oxides (O2-), using carbon (C) as the reducing agent. The reduction is usually conducted in the electric arc furnace or reverberatory furnace, depending on the metal ore. These chemical reactions are usually conducted at temperatures of several hundred degrees Celsius. Such processes are applied for production of the elemental forms of many elements. The ability of metals to participate in carbothermic reactions can be predicted from Ellingham diagrams.
Water-reactive substances are those that spontaneously undergo a chemical reaction with water, as they are highly reducing in nature. Notable examples include alkali metals, lithium through caesium, and alkaline earth metals, magnesium through barium.

A Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
Basic oxides are oxides that show basic properties, in opposition to acidic oxides. A basic oxide can either react with water to form a base, or with an acid to form a salt and water in a neutralization reaction.
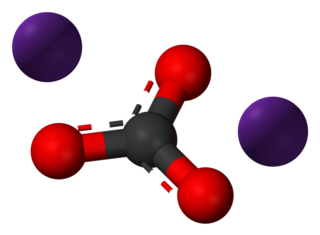
Caesium carbonate or cesium carbonate is a chemical compound with the chemical formula Cs2CO3. It is white crystalline solid. Caesium carbonate has a high solubility in polar solvents such as water, ethanol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium carbonate and sodium carbonate, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.
Suboxides are a class of oxides wherein the electropositive element is in excess relative to the “normal” oxides. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters.



















