An electron and an electron hole that are attracted to each other by the Coulomb force can form a bound state called an exciton. It is an electrically neutral quasiparticle that exists mainly in condensed matter, including insulators, semiconductors, some metals, but also in certain atoms, molecules and liquids. The exciton is regarded as an elementary excitation that can transport energy without transporting net electric charge.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.

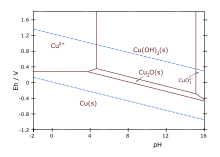
Basic copper carbonate is a chemical compound, more properly called copper(II) carbonate hydroxide. It is an ionic compound consisting of the ions copper(II) Cu2+
, carbonate CO2−
3, and hydroxide OH−
.

Copper oxide is any of several binary compounds composed of the elements copper and oxygen. Two oxides are well known, Cu2O and CuO, corresponding to the minerals cuprite and tenorite, respectively. Paramelaconite (Cu4O3) is less well characterized.

Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Tin(II) oxide is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.
Copper(I) hydroxide is the hydroxide of the metal copper with the chemical formula of CuOH. It is a mild, highly unstable alkali. The color of pure CuOH is yellow or orange-yellow, but it usually appears rather dark red because of impurities. It is extremely easily oxidized even at room temperature. It is useful for some industrial processes and in preventing condensation of formaldehyde. It is also an important reactant and intermediate for several important products including Cu2O3 and Cu(OH)2. Additionally, it can act as a catalyst in the synthesis pyrimidopyrrolidone derivatives.

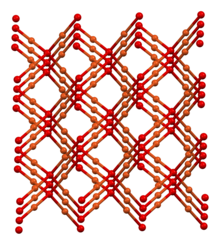
Delafossite is a copper iron oxide mineral with formula CuFeO2 or Cu1+Fe3+O2. It is a member of the delafossite mineral group, which has the general formula ABO2, a group characterized by sheets of linearly coordinated A cations stacked between edge-shared octahedral layers (BO6). Delafossite, along with other minerals of the ABO2 group, is known for its wide range of electrical properties, its conductivity varying from insulating to metallic. Delafossite is usually a secondary mineral that crystallizes in association with oxidized copper and rarely occurs as a primary mineral.
Copper(I) sulfate, also known as cuprous sulfate, is an inorganic compound with the chemical formula Cu2SO4. It is a white solid, in contrast to copper(II) sulfate, which is blue in hydrous form. Compared to the commonly available reagent, copper(II) sulfate, copper(I) sulfate is unstable and not readily available.

Paramelaconite is a rare, black-colored copper(I,II) oxide mineral with formula CuI
2CuII
2O3 (or Cu4O3). It was discovered in the Copper Queen Mine in Bisbee, Arizona, about 1890. It was described in 1892 and more fully in 1941. Its name is derived from the Greek word for "near" and the similar mineral melaconite, now known as tenorite.
Bose–Einstein condensation can occur in quasiparticles, particles that are effective descriptions of collective excitations in materials. Some have integer spins and can be expected to obey Bose–Einstein statistics like traditional particles. Conditions for condensation of various quasiparticles have been predicted and observed. The topic continues to be an active field of study.
Copper peroxide is a hypothetical inorganic compound with the chemical formula CuO2. The 1:2 ratio of copper and oxygen would be consistent with copper in its common +2 oxidation state and a peroxide group. Although samples of this composition have not been isolated, CuO2 has attracted interest from computational perspective. One highly cited analysis concludes that gaseous CuO2 is a superoxide, with copper in a +1 oxidation state: Cu+O−2.

Copper(I) thiocyanate is a coordination polymer with formula CuSCN. It is an air-stable, white solid used as a precursor for the preparation of other thiocyanate salts.

Chevreul's salt (copper(I,II) sulfite dihydrate, Cu2SO3•CuSO3•2H2O or Cu3(SO3)2•2H2O), is a copper salt which was prepared for the first time by a French chemist Michel Eugène Chevreul in 1812. Its unusual property is that it contains copper in both of its common oxidation states, making it a mixed-valence complex. It is insoluble in water and stable in air. What was known as Rogojski's salt is a mixture of Chevreul's salt and metallic copper.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.