Related Research Articles

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Redox is a type of chemical reaction in which the oxidation states of a reactant change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula O−2. The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide".

Titanic acid is a general name for a family of chemical compounds of the elements titanium, hydrogen, and oxygen, with the general formula [TiOx(OH)4−2x]n. Various simple titanic acids have been claimed, mainly in the older literature. No crystallographic and little spectroscopic support exists for these materials. Some older literature refers to TiO2 as titanic acid, and the dioxide forms an unstable hydrate when TiCl4 hydrolyzes.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO). Cuprous oxide is a red-coloured solid and is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles. Copper(I) oxide is found as the reddish mineral cuprite.
Cuprates are a class of compounds that contain copper (Cu) atom(s) in an anion. They can be broadly categorized into two main types:

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.
Fenton's reagent is a solution of hydrogen peroxide (H2O2) and an iron catalyst (typically iron(II) sulfate, FeSO4). It is used to oxidize contaminants or waste water as part of an advanced oxidation process. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene and tetrachloroethylene (perchloroethylene). It was developed in the 1890s by Henry John Horstman Fenton as an analytical reagent.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O. The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.
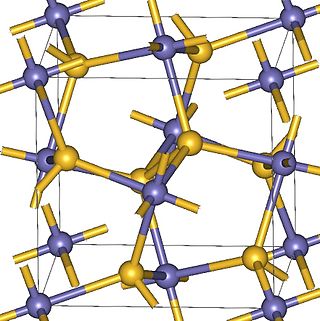
Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc peroxide has also been used as an oxidant in explosives and pyrotechnic mixtures. Its properties have been described as a transition between ionic and covalent peroxides. Zinc peroxide can be synthesized through the reaction of zinc chloride and hydrogen peroxide.

Schweizer's reagent is a metal ammine complex with the formula [Cu(NH3)4(H2O)2](OH)2. This deep-blue compound is used in purifying cellulose. This salt consists of tetraamminediaquacopper(II) cations and hydroxide anions.
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.

The blue bottle experiment is a color-changing redox chemical reaction. An aqueous solution containing glucose, sodium hydroxide, methylene blue is prepared in a closed bottle containing some air. Upon standing, it spontaneously turns from blue to colorless due to reduction of methylene blue by the alkaline glucose solution. However, shaking the bottle oxidizes methylene blue back into its blue form. With further shaking, this color-change cycle can be repeated many times. This experiment is a classic chemistry demonstration that can be used in laboratory courses as a general chemistry experiment to study chemical kinetics and reaction mechanism. The reaction also works with other reducing agents besides glucose and other redox indicator dyes besides methylene blue.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.
References
- ↑ Gutsev, G. L.; Rao, B. K.; Jena, P. (2000). "Systematic Study of Oxo, Peroxo, and Superoxo Isomers of 3d-Metal Dioxides and Their Anions". The Journal of Physical Chemistry A. 104 (51): 11961–11971. Bibcode:2000JPCA..10411961G. doi:10.1021/jp002252s.
- 1 2 The Collected Works of Sir Humphry Davy: Discourses delivered before the Royal society. Elements of agricultural chemistry, pt. I. The Chemical Society (Great Britain). 1894. p. 32.
- ↑ Krüss, Gerhard (1884). "Einige Beobachtungen über die höheren Sauerstoffverbindungen des Kupfers" (abstract). Ber. 17 (2): 2593–2597. doi:10.1002/cber.188401702186.
- ↑ Elwell, Courtney E.; Gagnon, Nicole L.; Neisen, Benjamin D.; Dhar, Debanjan; Spaeth, Andrew D.; Yee, Gereon M.; Tolman, William B. (2017). "Copper–Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity". Chemical Reviews. 117 (3): 2059–2107. doi:10.1021/acs.chemrev.6b00636. PMC 5963733 . PMID 28103018.