
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains. Anhydrous copper sulfate is a light grey powder.

Thiocyanates are salts containing the thiocyanate anion [SCN]−. [SCN]− is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.
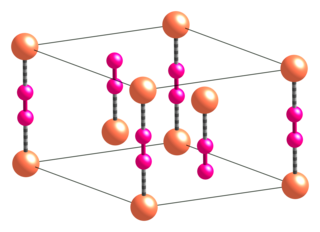
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.

Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur:

Thiocyanogen, (SCN)2, is a pseudohalogen derived from the pseudohalide thiocyanate, [SCN]−. This hexatomic compound exhibits C2 point group symmetry and has the connectivity NCS-SCN. The oxidation ability is greater than bromine. It reacts with water:

Cobalt(II) thiocyanate is an inorganic compound with the formula Co(SCN)2. The anhydrous compound is a coordination polymer with a layered structure. The trihydrate, Co(SCN)2(H2O)3, is a isothiocyanate complex used in the cobalt thiocyanate test (or Scott test) for detecting cocaine. The test has been responsible for widespread false positives and false convictions.

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.

The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction was invented by and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. There are three generations of this reaction, with the first generation shown below. The original transformation used catalytic Pd(0), TFP = tris(2-furyl)phosphine as an additional ligand and stoichiometric CuTC = copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.

Silver thiocyanate is the silver salt of thiocyanic acid with the formula AgSCN. Silver thiocyanate appears as a white crystalline powder. It is very commonly used in the synthesis of silver nanoparticles. Additionally, studies have found silver nanoparticles to be present in saliva present during the entire digestive process of silver nitrate. Silver thiocyanate is slightly soluble in water, with a solubility of 1.68 x 10−4 g/L. It is insoluble in ethanol, acetone, and acid.

Copper(I) thiocyanate is a coordination polymer with formula CuSCN. It is an air-stable, white solid used as a precursor for the preparation of other thiocyanate salts.

Nickel(II) thiocyanate is a coordination polymer with formula Ni(SCN)2. It is a green-brown solid and its crystal structure was determined first in 1982.
Sulfidostannates, or thiostannates are chemical compounds containing anions composed of tin linked with sulfur. They can be considered as stannates with sulfur substituting for oxygen. Related compounds include the thiosilicates, and thiogermanates, and by varying the chalcogen: selenostannates, and tellurostannates. Oxothiostannates have oxygen in addition to sulfur. Thiostannates can be classed as chalcogenidometalates, thiometallates, chalcogenidotetrelates, thiotetrelates, and chalcogenidostannates. Tin is almost always in the +4 oxidation state in thiostannates, although a couple of mixed sulfides in the +2 state are known,

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Lithium thiocyanate is a chemical compound with the formula LiSCN. It is an extremely hygroscopic white solid that forms the monohydrate and the dihydrate. It is the least stable of the alkali metal thiocyanates due to the large electrostatic deforming field of the lithium cation.
Transition metal complexes of thiocyanate describes coordination complexes containing one or more thiocyanate (SCN-) ligands. The topic also includes transition metal complexes of isothiocyanate. These complexes have few applications but played significant role in the development of coordination chemistry.















