Antimony is a chemical element with the symbol Sb (from Latin stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of the metalloid in the West was written in 1540 by Vannoccio Biringuccio.
The Ostwald process is a chemical process used for making nitric acid (HNO3). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. Historically and practically, the Ostwald process is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3).

Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely-used stain in electron microscopy.

Nitrogen dioxide is a chemical compound with the formula NO2. It is one of several nitrogen oxides. NO
2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry.
In chemistry, antimonite refers to a salt of antimony(III), such as NaSb(OH)4 and NaSbO2 (meta-antimonite), which can be prepared by reacting alkali with antimony trioxide, Sb2O3. These are formally salts of antimonous acid, Sb(OH)3, whose existence in solution is dubious. Attempts to isolate it generally form Sb2O3·xH2O, antimony(III) oxide hydrate, which slowly transforms into Sb2O3.

Antimony trisulfide is found in nature as the crystalline mineral stibnite and the amorphous red mineral metastibnite. It is manufactured for use in safety matches, military ammunition, explosives and fireworks. It also is used in the production of ruby-colored glass and in plastics as a flame retardant. Historically the stibnite form was used as a grey pigment in paintings produced in the 16th century. In 1817, the dye and fabric chemist, John Mercer discovered the non-stoichiometric compound Antimony Orange, the first good orange pigment available for cotton fabric printing.
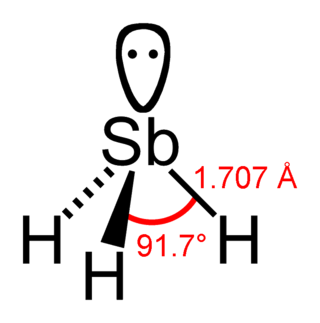
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs).

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.

Antimony pentoxide (molecular formula: Sb2O5) is a chemical compound of antimony and oxygen. It contains antimony in the +5 oxidation state.

Antimony trichloride is the chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as butter of antimony.

Antimony triiodide is the chemical compound with the formula SbI3. This ruby-red solid is the only characterized "binary" iodide of antimony, i.e. the sole compound isolated with the formula SbxIy. It contains antimony in its +3 oxidation state. Like many iodides of the heavier main group elements, its structure depends on the phase. Gaseous SbI3 is a molecular, pyramidal species as anticipated by VSEPR theory. In the solid state, however, the Sb center is surrounded by an octahedron of six iodide ligands, three of which are closer and three more distant. For the related compound BiI3, all six Bi—I distances are equal.
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:

Antimony(III) acetate is the compound of antimony with the chemical formula of Sb(CH3CO2)3. It is a white powder, is moderately water-soluble, and is used as a catalyst in the production of polyesters.
Stock nomenclature for inorganic compounds is a widely used system of chemical nomenclature developed by the German chemist Alfred Stock and first published in 1919. In the "Stock system", the oxidation states of some or all of the elements in a compound are indicated in parentheses by Roman numerals.

Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.