In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide.
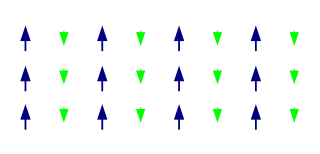
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when the populations consist of different atoms or ions (such as Fe2+ and Fe3+).

Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 is α polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Calcium nitride is the inorganic compound with the chemical formula Ca3N2. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered.
Solid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K (−218.79 °C, −361.82 °F). Solid oxygen O2, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red part of the visible light spectrum.

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).

Gallium(III) oxide is an inorganic compound and ultra-wide bandgap semiconductor with the formula Ga2O3. It is actively studied for applications in power electronics, phosphors, and gas sensing. The compound has several polymorphs, of which the monoclinic β-phase is the most stable. The β-phase’s bandgap of 4.7–4.9 eV and large-area, native substrates make it a promising competitor to GaN and SiC-based power electronics applications and solar-blind UV photodetectors. Ga2O3 exhibits reduced thermal conductivity and electron mobility by an order of magnitude compared to GaN and SiC, but is predicted to be significantly more cost-effective due to being the only wide-bandgap material capable of being grown from melt. β-Ga2O3 is thought to be radiation hard which makes it promising for military and space applications.

At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe), gamma iron (γ-Fe), and delta iron (δ-Fe). At very high pressure, a fourth form exists, called epsilon iron (ε-Fe). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures.

Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.

Manganese(II) oxide is an inorganic compound with chemical formula MnO. It forms green crystals. The compound is produced on a large scale as a component of fertilizers and food additives.

Iron oxide nanoparticles are iron oxide particles with diameters between about 1 and 100 nanometers. The two main forms are magnetite and its oxidized form maghemite. They have attracted extensive interest due to their superparamagnetic properties and their potential applications in many fields.

Managnese(II) molybdate is an inorganic compound with the chemical formula MnMoO4. α-MnMoO4 has a monoclinic crystal structure. It is also antiferromagnetic at low temperatures.
Nickel(II) titanate is an inorganic compound with the chemical formula NiTiO3 nickel(II) titanate, also known as nickel titanium oxide, is a coordination compound between nickel(II), titanium(IV) and oxide ions. It has the appearance of a yellow powder. There are several methods of synthesis for nickel(II) titanate. The first method involves nickel(II) titanate's melting temperature of over 500 °C at which its precursor decomposes to give nickel(II) titanate as a residue. Nickel(II) titanate has been used as a catalyst for toluene oxidation. The second method involved using enthalpy and entropy on the reaction to synthesize nickel(II) titanate through its phase transition.
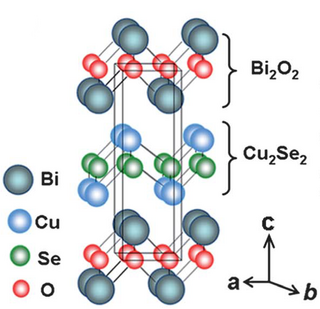
Oxyselenides are a group of chemical compounds that contain oxygen and selenium atoms. Oxyselenides can form a wide range of structures in compounds containing various transition metals, and thus can exhibit a wide range of properties. Most importantly, oxyselenides have a wide range of thermal conductivity, which can be controlled with changes in temperature in order to adjust their thermoelectric performance. Current research on oxyselenides indicates their potential for significant application in electronic materials.

Lithium iridate, Li2IrO3, is a chemical compound of lithium, iridium and oxygen. It forms black crystals with three slightly different layered atomic structures, α, β, and sometimes γ. Lithium iridate exhibits metal-like, temperature-independent electrical conductivity, and changes its magnetic ordering from paramagnetic to antiferromagnetic upon cooling to 15 K.
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.
Manganese oxalate is a chemical compound, a salt of manganese and oxalic acid with the chemical formula MnC
2O
4. The compound creates light pink crystals, does not dissolve in water, and forms crystalline hydrates. It occurs naturally as the mineral Lindbergite.
Corundum is the name for a structure prototype in inorganic solids, derived from the namesake polymorph of aluminum oxide (α-Al2O3). Other compounds, especially among the inorganic solids, exist in corundum structure, either in ambient or other conditions. Corundum structures are associated with metal-insulator transition, ferroelectricity, polar magnetism, and magnetoelectric effects.