Praseodymium is a chemical element with the symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.

Praseodymium(III) oxide, praseodymium oxide or praseodymia is the chemical compound composed of praseodymium and oxygen with the formula Pr2O3. It forms light green hexagonal crystals. Praseodymium(III) oxide crystallizes in the manganese(III) oxide or bixbyite structure.
Praseodymium oxide may refer to:
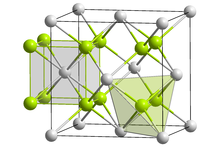
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.
Terbium(IV) oxide is an inorganic compound with a chemical formula TbO2. It can be produced by oxidizing terbium(III) oxide by oxygen gas at 1000 atm and 300 °C.

Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble in water but soluble in concentrated acids.
Praseodymium(III) hydroxide is an inorganic compound with a chemical formula Pr(OH)3.
Praseodymium(III) oxalate is an inorganic compound, a salt of praseodymium metal and oxalic acid with the chemical formula C6O12Pr2. The compound forms light green crystals, insoluble in water, also forms crystalline hydrates.

Praseodymium(IV) fluoride (also praseodymium tetrafluoride) is a binary inorganic compound, a highly oxidised metal salt of praseodymium and fluoride with the chemical formula PrF4.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Praseodymium arsenate is the arsenate salt of praseodymium, with the chemical formula of PrAsO4. It has good thermal stability. Its ferroelectric transition temperature is 52°C.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Praseodymium(III) perchlorate is the perchlorate salt of praseodymium, with the chemical formula of Pr(ClO4)3.
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.

Praseodymium(V) oxide nitride is a compound of praseodymium in the oxidation state of +5 with the formula PrNO, which was first reported in 2000, however, the compound wasn't verified to have an oxidation state of +5 until 2017. This compound is produced by the reaction of praseodymium metal and nitric oxide in 4K and solid neon. The crystal structure is linear with the praseodymium forming a triple bond with the nitrogen and a double bond with the oxygen. Calculation shows a significant level of f-orbital covalence of Pr-X bonds.
Praseodymium orthoscandate is a chemical compound, a rare-earth oxide with a perovskite structure. It has the chemical formula of PrScO3.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.