
Neodymium is a chemical element; it has symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is the case with many other rare-earth metals.

Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Yttrium aluminium garnet (YAG, Y3Al5O12) is a synthetic crystalline material of the garnet group. It is a cubic yttrium aluminium oxide phase, with other examples being YAlO3 (YAP) in a hexagonal or an orthorhombic, perovskite-like form, and the monoclinic Y4Al2O9 (YAM).
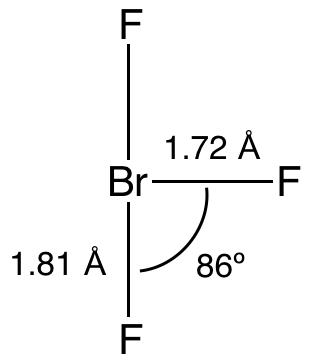
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

Cerium is a chemical element; it has symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.

Cerium(III) fluoride (or cerium trifluoride), CeF3, is an ionic compound of the rare earth metal cerium and fluorine.

Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.

Samarium(III) nitrate is an odorless, white-colored chemical compound with the formula Sm(NO3)3. It forms the hexahydrate, which decomposes at 50°C to the anhydrous form. When further heated to 420°C, it is converted to the oxynitrate, and at 680°C it decomposes to form samarium(III) oxide.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
Bromosyl trifluoride is an inorganic compound of bromine, fluorine, and oxygen with the chemical formula BrOF3.

Praseodymium arsenide is a binary inorganic compound of praseodymium and arsenic with the formula PrAs.















