Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. This group is sometimes called the vanadium group or vanadium family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

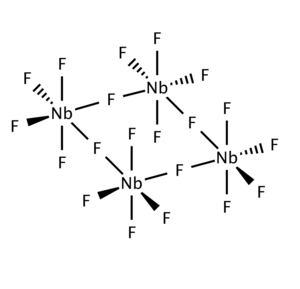
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.
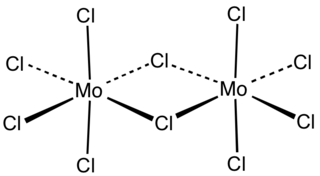
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Tantalum(V) fluoride is the inorganic compound with the formula TaF5. It is one of the principal molecular compounds of tantalum. Characteristic of some other pentafluorides, the compound is volatile but exists as an oligomer in the solid state.

Gold(V) fluoride is the inorganic compound with the formula Au2F10. This fluoride compound features gold in its highest known oxidation state. This red solid dissolves in hydrogen fluoride but these solutions decompose, liberating fluorine.
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2.

Niobium pentoxide is the inorganic compound with the formula Nb2O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.
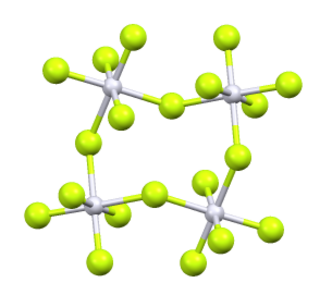
Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.

Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is highly sensitive to air and moisture, and disproportiates into niobium(III) chloride and niobium(V) chloride when heated.
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.

Niobium oxychloride is the inorganic compound with the formula NbOCl3. It is a white, crystalline, diamagnetic solid. It is often found as an impurity in samples of niobium pentachloride, a common reagent in niobium chemistry.
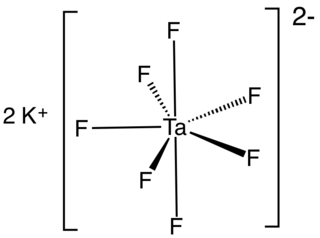
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.

Vanadium(V) chloride is the inorganic compound with the formula VCl5. It is a black diamagnetic solid. The molecules adopt a bioctahedral structure similar to that of niobium(V) chloride.

Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.
Niobium(III) chloride also known as niobium trichloride is a compound of niobium and chlorine. The binary phase NbCl3 is not well characterized but many adducts are known.