
Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:

Diethylaminosulfur trifluoride (DAST) is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds. The compound is colourless; older samples assume an orange colour.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.

Thionyl tetrafluoride, also known as sulfur tetrafluoride oxide, is an inorganic compound with the formula SOF4. It is a colorless gas.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Sulfur chloride pentafluoride is an inorganic compound with the formula SF5Cl. It exists as a colorless gas at room temperature and is highly toxic, like most inorganic compounds containing the pentafluorosulfide functional group. The compound adopts an octahedral geometry with C
4v symmetry. Sulfur chloride pentafluoride is the only commercially available reagent for adding the –SF5 group to organic compounds.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.
Phosphoryl fluoride is a compound with the chemical formula POF3. It is a colorless gas that hydrolyzes rapidly. It has a critical temperature of 73 °C and a critical pressure of 4.25 bars.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.

Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO2F−2. It has a single negative charge and resembles perchlorate and monofluorosulfonate in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.
Difluoroamino sulfur pentafluoride is a gaseous chemical compound of fluorine, sulfur, and nitrogen. It is unusual in having a hexa-coordinated sulfur atom with a link to nitrogen. Other names for this substance include difluoro(pentafluorosulfur)amine, pentafluorosulfanyldifluoramine, and pentafluorosulfanyl N,N-difluoramine.
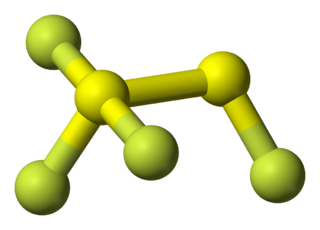
1,1,1,2-tetrafluorodisulfane, also known as 1,2-difluorodisulfane 1,1-difluoride or just difluorodisulfanedifluoride (FSSF3) is an unstable molecular compound of fluorine and sulfur. The molecule has a pair of sulfur atoms, with one fluorine atom on one sulfur, and three fluorine atoms on the other. It has the uncommon property that all the bond lengths are different. The bond strength is not correlated with bond length but is inversely correlated with the force constant (Badger's rule). The molecule can be considered as sulfur tetrafluoride in which a sulfur atom is inserted into a S-F bond.
Pentafluorosulfur hypofluorite is an oxyfluoride of sulfur in the +6 oxidation state, with a fluorine atom attached to oxygen. The formula is SOF6. In standard conditions it is a gas.
Diphosphorus tetrafluoride is a gaseous compound of phosphorus and fluorine with formula P2F4. Two fluorine atoms are connected to each phosphorus atom, and there is a bond between the two phosphorus atoms. Phosphorus can be considered to have oxidation state +2, as indicated by the name phosphorus difluoride.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. It can be produced by the reaction between very pure terbium(III) fluoride and xenon difluoride, chlorine trifluoride or fluorine gas:














