
An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 or less commonly group 5 and group 7 transition metals in their high oxidation states. Polyoxometalates are often colorless, orange or red diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one metal, oxide, and a main group oxyanion. Many exceptions to these general statements exist.

Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.
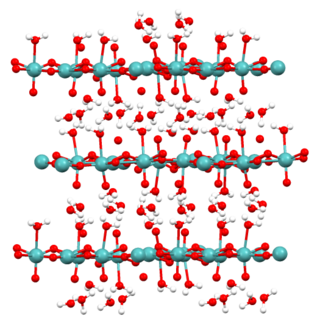
Molybdic acid refers to hydrated forms of molybdenum trioxide and related species. The monohydrate (MoO3·H2O) and the dihydrate (MoO3·2H2O) are well characterized. They are yellow diamagnetic solids.

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.
Metal aromaticity or metalloaromaticity is the concept of aromaticity, found in many organic compounds, extended to metals and metal-containing compounds. The first experimental evidence for the existence of aromaticity in metals was found in aluminium cluster compounds of the type MAl−
4 where M stands for lithium, sodium or copper. These anions can be generated in a helium gas by laser vaporization of an aluminium / lithium carbonate composite or a copper or sodium / aluminium alloy, separated and selected by mass spectrometry and analyzed by photoelectron spectroscopy. The evidence for aromaticity in these compounds is based on several considerations. Computational chemistry shows that these aluminium clusters consist of a tetranuclear Al2−
4 plane and a counterion at the apex of a square pyramid. The Al2−
4 unit is perfectly planar and is not perturbed the presence of the counterion or even the presence of two counterions in the neutral compound M
2Al
4. In addition its HOMO is calculated to be a doubly occupied delocalized pi system making it obey Hückel's rule. Finally a match exists between the calculated values and the experimental photoelectron values for the energy required to remove the first 4 valence electrons. The first fully metal aromatic compound was a cyclogallane with a Ga32- core discovered by Gregory Robinson in 1995.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.

Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula Mn2O7. Manganese heptoxide is a volatile liquid with an oily consistency. It is a highly reactive and powerful oxidizer that reacts explosively with nearly any organic compound. It was first described in 1860. It is the acid anhydride of permanganic acid.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).

Molybdenum blue is a term applied to:

Dichlorine hexoxide is the chemical compound with the molecular formula Cl
2O
6, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate [ClO
2]+
[ClO
4]−
, which may be thought of as the mixed anhydride of chloric and perchloric acids.
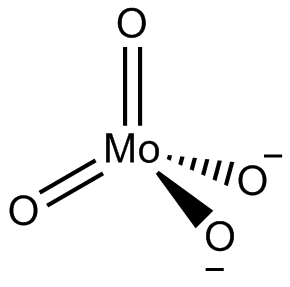
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.

Manganese(II) molybdate is an inorganic compound with the chemical formula MnMoO4. α-MnMoO4 has a monoclinic crystal structure. It is also antiferromagnetic at low temperatures.
Ammonium dimolybdate (ADM) is the inorganic compound with the formula (NH4)2Mo2O7. It is a white, water-soluble solid. ADM is an intermediate in the production of molybdenum compounds from its ores. Roasting typical ore produces crude molybdenum(VI) oxides, which can be extracted into aqueous ammonia, affording ammonium molybdate. Heating solutions of ammonium molybdate gives ADM. Upon heating, solid ammonium dimolybdate decomposes to molybdenum trioxide:
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.


















