Related Research Articles

Polonium is a chemical element with the symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though slightly longer-lived isotopes exist, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.
The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
In chemistry, perxenates are salts of the yellow xenon-containing anion XeO4−
6. This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°. The Xe–O bond length was determined by X-ray crystallography to be 1.875 Å.
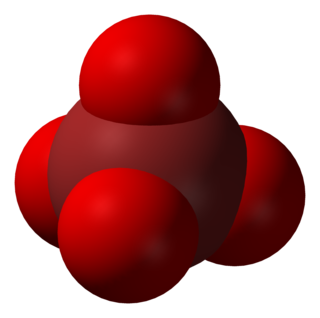
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
In chemistry, a plumbate is a salt having one of the several lead-containing oxoanions. Although the term plumbate can refer either to plumbate(II) or plumbate(IV), it traditionally refers specifically to plumbate(IV), whereas plumbate(II) is referred to as plumbite.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.
Xenic acid is a noble gas compound formed by the dissolution of xenon trioxide in water. Its chemical formula is H2XeO4. It is a very powerful oxidizing agent, and its decomposition is dangerous as it liberates a large amount of gaseous products: xenon, oxygen, and ozone. However, this feature is also what makes xenic acid practically useful in syntheses: there is no chance of introducing impurities to the oxidation products, as all the byproducts can be trivially evaporated.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.

Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. It is a white, waxy, crystalline and highly toxic solid with garlic odour.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Dinitrogen trioxide is the chemical compound with the formula N2O3. This deep blue solid is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
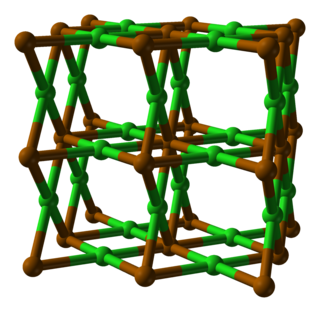
Polonium dichloride is a chemical compound of the radioactive metalloid,polonium and chlorine. Its chemical formula is PoCl2.
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen; like all polonium compounds, it is highly radioactive. It is a volatile and very labile compound, from which many polonides can be derived.
Polonium hexafluoride (PoF6) is a possible chemical compound of polonium and fluorine and one of the seventeen known binary hexafluorides.
The chalcogens react with each other to form interchalcogen compounds.
Polonium tetrachloride (also known as polonium(IV) chloride) is a chemical compound with the formula PoCl4. It is a hygroscopic bright yellow crystalline solid at room temperature. Above 200 °C, it tends to decompose into polonium dichloride and excess chlorine, similar to selenium tetrachloride and tellurium tetrachloride.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Polonium monoxide (also known as polonium(II) oxide) is a chemical compound with the formula PoO. It is one of three oxides of polonium, the other two being polonium dioxide (PoO2) and polonium trioxide (PoO3). It is an interchalcogen.
Polonium dibromide (also known as polonium(II) bromide) is a chemical compound with the formula PoBr2. It is a purple-brown crystalline solid at room temperature. It sublimes (decomposing slightly) at 110 °C/30 μ and decomposes when melted in nitrogen gas at 270–280 °C.
References
- ↑ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 594, ISBN 0-12-352651-5
- 1 2 3 4 Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046 . Retrieved June 14, 2012.
- 1 2 Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. 10. p. 78. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.