
An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

Carbon nanofibers (CNFs), vapor grown carbon fibers (VGCFs), or vapor grown carbon nanofibers (VGCNFs) are cylindrical nanostructures with graphene layers arranged as stacked cones, cups or plates. Carbon nanofibers with graphene layers wrapped into perfect cylinders are called carbon nanotubes.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.

Tungsten disulfide is an inorganic chemical compound composed of tungsten and sulfur with the chemical formula WS2. This compound is part of the group of materials called the transition metal dichalcogenides. It occurs naturally as the rare mineral tungstenite. This material is a component of certain catalysts used for hydrodesulfurization and hydrodenitrification.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.
As the world's energy demand continues to grow, the development of more efficient and sustainable technologies for generating and storing energy is becoming increasingly important. According to Dr. Wade Adams from Rice University, energy will be the most pressing problem facing humanity in the next 50 years and nanotechnology has potential to solve this issue. Nanotechnology, a relatively new field of science and engineering, has shown promise to have a significant impact on the energy industry. Nanotechnology is defined as any technology that contains particles with one dimension under 100 nanometers in length. For scale, a single virus particle is about 100 nanometers wide.

Niobium pentoxide is the inorganic compound with the formula Nb2O5. A colorless, insoluble, and fairly unreactive solid, it is the most widespread precursor for other compounds and materials containing niobium. It is predominantly used in alloying, with other specialized applications in capacitors, optical glasses, and the production of lithium niobate.

Pseudocapacitors store electrical energy faradaically by electron charge transfer between electrode and electrolyte. This is accomplished through electrosorption, reduction-oxidation reactions, and intercalation processes, termed pseudocapacitance.
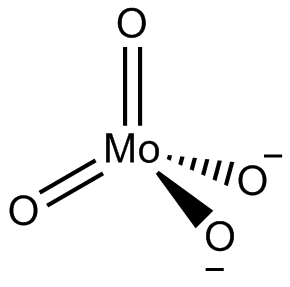
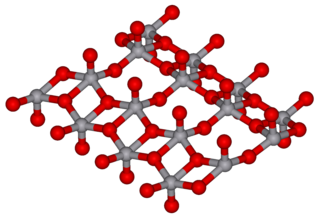
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
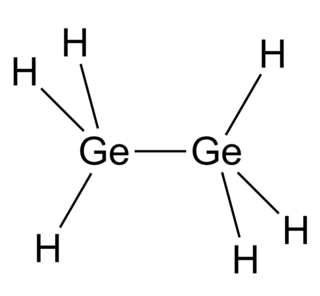
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. They are analogous to metal nitrido complexes. Carbido complexes are a molecular subclass of carbides, which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis, and related catalytic industrial processes. Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +2, +3, and +4. Well-characterized compounds containing iridium in the +6 oxidation state include IrF6 and the oxides Sr2MgIrO6 and Sr2CaIrO6. iridium(VIII) oxide was generated under matrix isolation conditions at 6 K in argon. The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO4]+.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.








