A nanowire is a nanostructure in the form of a wire with the diameter of the order of a nanometre. More generally, nanowires can be defined as structures that have a thickness or diameter constrained to tens of nanometers or less and an unconstrained length. At these scales, quantum mechanical effects are important—which coined the term "quantum wires".
Indium tin oxide (ITO) is a ternary composition of indium, tin and oxygen in varying proportions. Depending on the oxygen content, it can be described as either a ceramic or an alloy. Indium tin oxide is typically encountered as an oxygen-saturated composition with a formulation of 74% In, 8% Sn, and 18% O by weight. Oxygen-saturated compositions are so typical that unsaturated compositions are termed oxygen-deficient ITO. It is transparent and colorless in thin layers, while in bulk form it is yellowish to gray. In the infrared region of the spectrum it acts as a metal-like mirror.
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, light-emitting diodes, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.

Delamination is a mode of failure where a material fractures into layers. A variety of materials, including laminate composites and concrete, can fail by delamination. Processing can create layers in materials, such as steel formed by rolling and plastics and metals from 3D printing which can fail from layer separation. Also, surface coatings, such as paints and films, can delaminate from the coated substrate.

Tantalum nitride (TaN) is a chemical compound, a nitride of tantalum. There are multiple phases of compounds, stoichimetrically from Ta2N to Ta3N5, including TaN.

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.

Thermal barrier coatings (TBCs) are advanced materials systems usually applied to metallic surfaces on parts operating at elevated temperatures, such as gas turbine combustors and turbines, and in automotive exhaust heat management. These 100 μm to 2 mm thick coatings of thermally insulating materials serve to insulate components from large and prolonged heat loads and can sustain an appreciable temperature difference between the load-bearing alloys and the coating surface. In doing so, these coatings can allow for higher operating temperatures while limiting the thermal exposure of structural components, extending part life by reducing oxidation and thermal fatigue. In conjunction with active film cooling, TBCs permit working fluid temperatures higher than the melting point of the metal airfoil in some turbine applications. Due to increasing demand for more efficient engines running at higher temperatures with better durability/lifetime and thinner coatings to reduce parasitic mass for rotating/moving components, there is significant motivation to develop new and advanced TBCs. The material requirements of TBCs are similar to those of heat shields, although in the latter application emissivity tends to be of greater importance.

Sputter deposition is a physical vapor deposition (PVD) method of thin film deposition by the phenomenon of sputtering. This involves ejecting material from a "target" that is a source onto a "substrate" such as a silicon wafer. Resputtering is re-emission of the deposited material during the deposition process by ion or atom bombardment. Sputtered atoms ejected from the target have a wide energy distribution, typically up to tens of eV. The sputtered ions can ballistically fly from the target in straight lines and impact energetically on the substrates or vacuum chamber. Alternatively, at higher gas pressures, the ions collide with the gas atoms that act as a moderator and move diffusively, reaching the substrates or vacuum chamber wall and condensing after undergoing a random walk. The entire range from high-energy ballistic impact to low-energy thermalized motion is accessible by changing the background gas pressure. The sputtering gas is often an inert gas such as argon. For efficient momentum transfer, the atomic weight of the sputtering gas should be close to the atomic weight of the target, so for sputtering light elements neon is preferable, while for heavy elements krypton or xenon are used. Reactive gases can also be used to sputter compounds. The compound can be formed on the target surface, in-flight or on the substrate depending on the process parameters. The availability of many parameters that control sputter deposition make it a complex process, but also allow experts a large degree of control over the growth and microstructure of the film.
High-power impulse magnetron sputtering is a method for physical vapor deposition of thin films which is based on magnetron sputter deposition. HIPIMS utilises extremely high power densities of the order of kW⋅cm−2 in short pulses (impulses) of tens of microseconds at low duty cycle of < 10%. Distinguishing features of HIPIMS are a high degree of ionisation of the sputtered metal and a high rate of molecular gas dissociation which result in high density of deposited films. The ionization and dissociation degree increase according to the peak cathode power. The limit is determined by the transition of the discharge from glow to arc phase. The peak power and the duty cycle are selected so as to maintain an average cathode power similar to conventional sputtering (1–10 W⋅cm−2).

A solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored, making it a potential alternative to batteries, methane, and other energy sources. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.

A copper indium gallium selenide solar cell is a thin-film solar cell used to convert sunlight into electric power. It is manufactured by depositing a thin layer of copper indium gallium selenide solid solution on glass or plastic backing, along with electrodes on the front and back to collect current. Because the material has a high absorption coefficient and strongly absorbs sunlight, a much thinner film is required than of other semiconductor materials.

Transparent conducting films (TCFs) are thin films of optically transparent and electrically conductive material. They are an important component in a number of electronic devices including liquid-crystal displays, OLEDs, touchscreens and photovoltaics. While indium tin oxide (ITO) is the most widely used, alternatives include wider-spectrum transparent conductive oxides (TCOs), conductive polymers, metal grids and random metallic networks, carbon nanotubes (CNT), graphene, nanowire meshes and ultra thin metal films.
Water oxidation is one of the half reactions of water splitting:

Platinum–iridium alloys are alloys of the platinum group precious metals platinum and iridium.
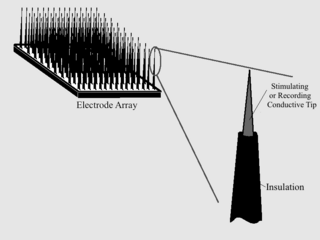
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.
High-entropy alloys (HEAs) are alloys that are formed by mixing equal or relatively large proportions of (usually) five or more elements. Prior to the synthesis of these substances, typical metal alloys comprised one or two major components with smaller amounts of other elements. For example, additional elements can be added to iron to improve its properties, thereby creating an iron-based alloy, but typically in fairly low proportions, such as the proportions of carbon, manganese, and others in various steels. Hence, high-entropy alloys are a novel class of materials. The term "high-entropy alloys" was coined by Taiwanese scientist Jien-Wei Yeh because the entropy increase of mixing is substantially higher when there is a larger number of elements in the mix, and their proportions are more nearly equal. Some alternative names, such as multi-component alloys, compositionally complex alloys and multi-principal-element alloys are also suggested by other researchers.

Lithium aluminium germanium phosphate, typically known with the acronyms LAGP or LAGPO, is an inorganic ceramic solid material whose general formula is Li
1+xAl
xGe
2-x(PO
4)
3. LAGP belongs to the NASICON family of solid conductors and has been applied as a solid electrolyte in all-solid-state lithium-ion batteries. Typical values of ionic conductivity in LAGP at room temperature are in the range of 10–5 - 10–4 S/cm, even if the actual value of conductivity is strongly affected by stoichiometry, microstructure, and synthesis conditions. Compared to lithium aluminium titanium phosphate (LATP), which is another phosphate-based lithium solid conductor, the absence of titanium in LAGP improves its stability towards lithium metal. In addition, phosphate-based solid electrolytes have superior stability against moisture and oxygen compared to sulfide-based electrolytes like Li
10GeP
2S
12 (LGPS) and can be handled safely in air, thus simplifying the manufacture process. Since the best performances are encountered when the stoichiometric value of x is 0.5, the acronym LAGP usually indicates the particular composition of Li
1.5Al
0.5Ge
1.5(PO
4)
3, which is also the typically used material in battery applications.
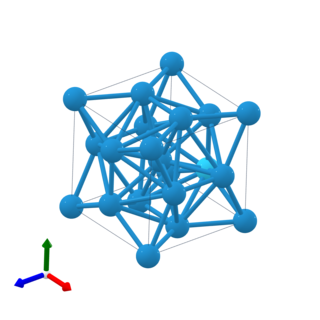
Beta-tungsten (β-W) is a metastable phase of tungsten widely observed in tungsten thin films. While the commonly existing stable alpha-tungsten (α-W) has a body-centered cubic (A2) structure, β-W adopts the topologically close-packed A15 structure containing eight atoms per unit cell, and it irreversibly transforms to the stable α phase through thermal annealing of up to 650 °C. It has been found that β-W possesses the giant spin Hall effect, wherein the applied charge current generates a transverse spin current, and this leads to potential applications in magnetoresistive random access memory devices.















