In particle physics, a lepton is an elementary particle of half-integer spin that does not undergo strong interactions. Two main classes of leptons exist: charged leptons, including the electron, muon, and tauon, and neutral leptons, better known as neutrinos. Charged leptons can combine with other particles to form various composite particles such as atoms and positronium, while neutrinos rarely interact with anything, and are consequently rarely observed. The best known of all leptons is the electron.

Pair production is the creation of a subatomic particle and its antiparticle from a neutral boson. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton. Pair production often refers specifically to a photon creating an electron–positron pair near a nucleus. As energy must be conserved, for pair production to occur, the incoming energy of the photon must be above a threshold of at least the total rest mass energy of the two particles created. Conservation of energy and momentum are the principal constraints on the process. All other conserved quantum numbers of the produced particles must sum to zero – thus the created particles shall have opposite values of each other. For instance, if one particle has electric charge of +1 the other must have electric charge of −1, or if one particle has strangeness of +1 then another one must have strangeness of −1.
The Bohr radius is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom. Its value is 5.29177210903(80)×10−11 m.
The hartree, also known as the Hartree energy, is the unit of energy in the atomic units system, named after the British physicist Douglas Hartree. Its CODATA recommended value is Eh = 4.3597447222071(85)×10−18 J = 27.211386245988(53) eV.
In atomic physics, the Bohr magneton is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum. In SI units, the Bohr magneton is defined as
In spectroscopy, the Rydberg constant, symbol for heavy atoms or for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants according to his model of the atom.
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, the chemical potential of any chemical species is uniformly the same everywhere throughout the system.
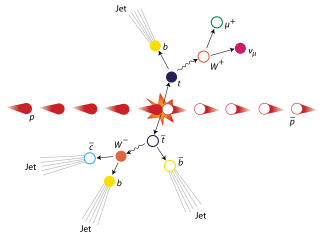
The top quark, sometimes also referred to as the truth quark, is the most massive of all observed elementary particles. It derives its mass from its coupling to the Higgs Boson. This coupling is very close to unity; in the Standard Model of particle physics, it is the largest (strongest) coupling at the scale of the weak interactions and above. The top quark was discovered in 1995 by the CDF and DØ experiments at Fermilab.
In particle physics, the W and Z bosons are vector bosons that are together known as the weak bosons or more generally as the intermediate vector bosons. These elementary particles mediate the weak interaction; the respective symbols are
W+
,
W−
, and
Z0
. The
W±
bosons have either a positive or negative electric charge of 1 elementary charge and are each other's antiparticles. The
Z0
boson is electrically neutral and is its own antiparticle. The three particles each have a spin of 1. The
W±
bosons have a magnetic moment, but the
Z0
has none. All three of these particles are very short-lived, with a half-life of about 3×10−25 s. Their experimental discovery was pivotal in establishing what is now called the Standard Model of particle physics.
The word "mass" has two meanings in special relativity: invariant mass is an invariant quantity which is the same for all observers in all reference frames, while the relativistic mass is dependent on the velocity of the observer. According to the concept of mass–energy equivalence, invariant mass is equivalent to rest energy, while relativistic mass is equivalent to relativistic energy.

In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude of torque the object experiences in a given magnetic field. When the same magnetic field is applied, objects with larger magnetic moments experience larger torques. The strength of this torque depends not only on the magnitude of the magnetic moment but also on its orientation relative to the direction of the magnetic field. Its direction points from the south pole to north pole of the magnet.
In physics, relativistic mechanics refers to mechanics compatible with special relativity (SR) and general relativity (GR). It provides a non-quantum mechanical description of a system of particles, or of a fluid, in cases where the velocities of moving objects are comparable to the speed of light c. As a result, classical mechanics is extended correctly to particles traveling at high velocities and energies, and provides a consistent inclusion of electromagnetism with the mechanics of particles. This was not possible in Galilean relativity, where it would be permitted for particles and light to travel at any speed, including faster than light. The foundations of relativistic mechanics are the postulates of special relativity and general relativity. The unification of SR with quantum mechanics is relativistic quantum mechanics, while attempts for that of GR is quantum gravity, an unsolved problem in physics.

In electrodynamics, the Larmor formula is used to calculate the total power radiated by a nonrelativistic point charge as it accelerates. It was first derived by J. J. Larmor in 1897, in the context of the wave theory of light.
Plasma parameters define various characteristics of a plasma, an electrically conductive collection of charged and neutral particles of various species that responds collectively to electromagnetic forces. Such particle systems can be studied statistically, i.e., their behaviour can be described based on a limited number of global parameters instead of tracking each particle separately.
Energy is defined via work, so the SI unit of energy is the same as the unit of work – the joule (J), named in honour of James Prescott Joule and his experiments on the mechanical equivalent of heat. In slightly more fundamental terms, 1 joule is equal to 1 newton metre and, in terms of SI base units
A g-factor is a dimensionless quantity that characterizes the magnetic moment and angular momentum of an atom, a particle or the nucleus. It is essentially a proportionality constant that relates the different observed magnetic moments μ of a particle to their angular momentum quantum numbers and a unit of magnetic moment, usually the Bohr magneton or nuclear magneton. Its value is proportional to the gyromagnetic ratio.
In strong-field laser physics, ponderomotive energy is the cycle-averaged quiver energy of a free electron in an electromagnetic field.
The Planck constant, or Planck's constant, denoted by , is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a matter wave equals the Planck constant divided by the associated particle momentum.
In particle physics, the electron mass is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about 9.109×10−31 kilograms or about 5.486×10−4 daltons, which has an energy-equivalent of about 8.187×10−14 joules or about 0.511 MeV.

In quantum electrodynamics, the Uehling potential describes the interaction potential between two electric charges which, in addition to the classical Coulomb potential, contains an extra term responsible for the electric polarization of the vacuum. This potential was found by Edwin Albrecht Uehling in 1935.