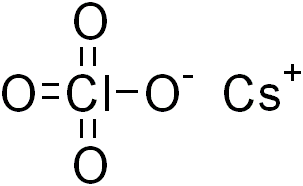The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Caesium is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.

Caesium hydroxide is a strong base containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide. It is the strongest of the five alkali metal hydroxides. Fused Caesium hydroxide dissolves glass by attacking silica framework and it has applications in bringing glass samples into a solution for analytical purposes in commercial glass industry and defense waste processing facility. The melting process is carried out in a nickel or zirconium crucible. Cesium hydroxide fusion at 750°C produces complete dissolution of glass pellets.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
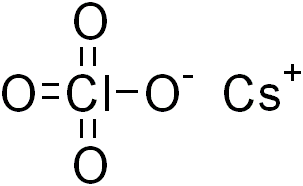
Caesium perchlorate or cesium perchlorate (CsClO4), is a perchlorate of caesium. It forms white crystals, which are sparingly soluble in cold water and ethanol. It dissolves more easily in hot water.

Caesium-137, cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from spontaneous fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. Caesium-137 has a relatively low boiling point of 671 °C (1,240 °F) and easily becomes volatile when released suddenly at high temperature, as in the case of the Chernobyl nuclear accident and with atomic explosions, and can travel very long distances in the air. After being deposited onto the soil as radioactive fallout, it moves and spreads easily in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. Caesium-137 was discovered by Glenn T. Seaborg and Margaret Melhase.

Uranyl zinc acetate (ZnUO2(CH3COO)4) is a compound of uranium.
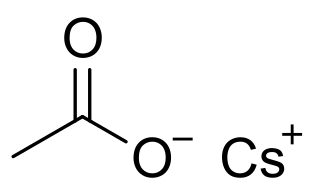
Caesium acetate or cesium acetate is an ionic caesium compound with the molecular formula CH3COOCs. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid.
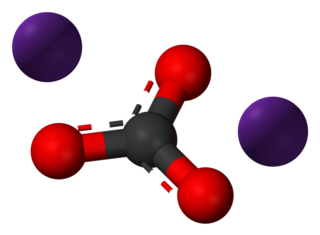
Caesium carbonate or cesium carbonate is a white crystalline solid compound. Caesium carbonate has a high solubility in polar solvents such as water, alcohol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium and sodium carbonates, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.
Caesium cadmium chloride (CsCdCl3) is a synthetic crystalline material. It belongs to the AMX3 group (where A=alkali metal, M=bivalent metal, X=halogen ions). It crystallizes in a hexagonal space group P63/mmc with unit cell lengths a = 7.403 Å and c = 18.406 Å, with one cadmium ion having D3d symmetry and the other having C3v symmetry.

Caesium nitrate or cesium nitrate is a salt with the chemical formula Cs N O3. An alkali metal nitrate, it is used in pyrotechnic compositions, as a colorant and an oxidizer, e.g. in decoys and illumination flares. The caesium emissions are chiefly due to two powerful spectral lines at 852.113 nm and 894.347 nm.
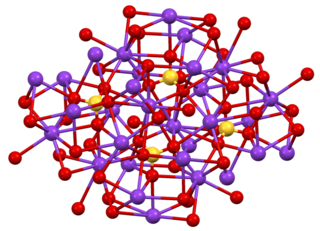
Caesium sulfate or cesium sulfate is the inorganic compound and salt with the formula Cs2SO4. It is a white water-soluble solid that is used to prepare dense aqueous solutions for use in isopycnic (or "density-gradient") centrifugation. It is isostructural with potassium salt.

Caesium oxalate (standard IUPAC spelling) dicesium oxalate, or cesium oxalate (American spelling) is the oxalate of caesium. Caesium oxalate has the chemical formula of Cs2C2O4.

Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.

Caesium peroxide or cesium peroxide is a compound of caesium and oxygen. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.
Caesium oxide, or cesium oxide, describes inorganic compounds composed of caesium and oxygen. Several binary oxides of caesium are known.

Rubidium selenide is an inorganic compound composed of selenium and rubidium. It is a selenide with a chemical formula of Rb2Se. Rubidium selenide is used together with caesium selenide in photovoltaic cells.

Caesium selenide is an inorganic compound of caesium and selenium. It is a selenide, with the chemical formula of Cs2Se. It can be prepared by reacting caesium and selenium. It has an inverse fluorite structure, with space group . There are 4 units per unit cell, and the other selenides from the same group are similar.