Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. Much of the current research on this salt is focused on its nanoparticles.

Hg1−xCdxTe or mercury cadmium telluride is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning the shortwave infrared to the very long wave infrared regions. The amount of cadmium (Cd) in the alloy can be chosen so as to tune the optical absorption of the material to the desired infrared wavelength. CdTe is a semiconductor with a bandgap of approximately 1.5 electronvolts (eV) at room temperature. HgTe is a semimetal, which means that its bandgap energy is zero. Mixing these two substances allows one to obtain any bandgap between 0 and 1.5 eV.
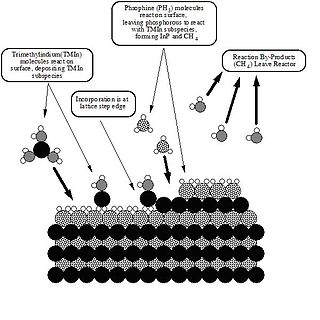
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures. As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation Science Center by Harold M. Manasevit.
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. It is a grey crystalline solid material.

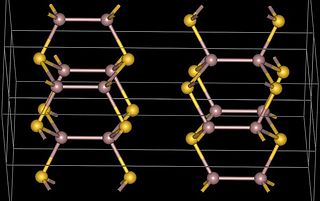
Bismuth telluride (Bi2Te3) is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. Bi2Te3 is a topological insulator, and thus exhibits thickness-dependent physical properties.
Lead telluride is a compound of lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite.

Dimethyl telluride is an organotelluride compound, formula (CH3)2Te, also known by the abbreviation DMTe.

Tin telluride is a compound of tin and tellurium (SnTe); is a IV-VI narrow band gap semiconductor and has direct band gap of 0.18 eV. It is often alloyed with lead to make lead tin telluride, which is used as an infrared detector material.

Mercury selenide (HgSe) is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm.

Germanium telluride (GeTe) is a chemical compound of germanium and tellurium and is a component of chalcogenide glasses. It shows semimetallic conduction and ferroelectric behaviour.
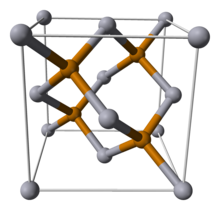
Gallium(II) telluride, GaTe, is a chemical compound of gallium and tellurium. There is research interest in the structure and electronic properties of GaTe because of the possibility that it, or related compounds, may have applications in the electronics industry. Gallium telluride can be made by reacting the elements or by metal organic vapour deposition (MOCVD). . GaTe produced from the elements has a monoclinic crystal structure. Each gallium atom is tetrahedrally coordinated by 3 tellurium and one gallium atom. The gallium-gallium bond length in the Ga2 unit is 2.43 Angstrom. The structure consists of layers and can be formulated as Ga24+ 2Te2−. The bonding within the layers is ionic-covalent and between the layers is predominantly van der Waals. GaTe is classified as a layered semiconductor (like GaSe and InSe which have similar structures). It is a direct band gap semiconductor with an energy of 1.65eV at room temperature. A hexagonal form can be produced by low pressure metal organic vapour deposition (MOCVD) from alkyl gallium telluride cubane-type clusters e.g. from (t-butylGa( μ3-Te))4. The core consists of a cube of eight atoms, four gallium, and four tellurium atoms. Each gallium has an attached t-butyl group and three adjacent tellurium atoms and each tellurium has three adjacent gallium atoms. The hexagonal form, which is closely related to the monoclinic form, containing Ga24+ units, converts to the monoclinic form when annealed at 500 °C.
The quantum spin Hall state is a state of matter proposed to exist in special, two-dimensional semiconductors that have a quantized spin-Hall conductance and a vanishing charge-Hall conductance. The quantum spin Hall state of matter is the cousin of the integer quantum Hall state, and that does not require the application of a large magnetic field. The quantum spin Hall state does not break charge conservation symmetry and spin- conservation symmetry.

A topological insulator is a material that behaves as an insulator in its interior but whose surface contains conducting states, meaning that electrons can only move along the surface of the material. Topological insulators have non-trivial symmetry-protected topological order; however, having a conducting surface is not unique to topological insulators, since ordinary band insulators can also support conductive surface states. What is special about topological insulators is that their surface states are symmetry-protected Dirac fermions by particle number conservation and time-reversal symmetry. In two-dimensional (2D) systems, this ordering is analogous to a conventional electron gas subject to a strong external magnetic field causing electronic excitation gap in the sample bulk and metallic conduction at the boundaries or surfaces.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.
A two-dimensional semiconductor is a type of natural semiconductor with thicknesses on the atomic scale. Geim and Novoselov et al. initiated the field in 2004 when they reported a new semiconducting material graphene, a flat monolayer of carbon atoms arranged in a 2D honeycomb lattice. A 2D monolayer semiconductor is significant because it exhibits stronger piezoelectric coupling than traditionally employed bulk forms. This coupling could enable applications. One research focus is on designing nanoelectronic components by the use of graphene as electrical conductor, hexagonal boron nitride as electrical insulator, and a transition metal dichalcogenide as semiconductor.

Molybdenum(IV) telluride, molybdenum ditelluride or just molybdenum telluride is a compound of molybdenum and tellurium with formula MoTe2, corresponding to a mass percentage of 27.32% molybdenum and 72.68% tellurium. It can crystallise in two dimensional sheets which can be thinned down to monolayers that are flexible and almost transparent. It is a semiconductor, and can fluoresce. It is part of a class of materials called transition metal dichalcogenides. As a semiconductor the band gap lies in the infrared region. This raises the potential use as a semiconductor in electronics or an infrared detector.
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.
Lithium telluride (Li2Te) is an inorganic compound of lithium and tellurium. Along with LiTe3, it is one of the two intermediate solid phases in the lithium-tellurium system. It can be prepared by directly reacting lithium and tellurium in a beryllium oxide crucible at 950°C.