
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης, meaning 'violet'.
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam in a cathode-ray tube.

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.

Lodewijk van den Berg was a Dutch-born American chemical engineer. He studied crystal growth and flew on a 1985 Space Shuttle Challenger mission as a payload specialist.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

A gamma-ray spectrometer (GRS) is an instrument for measuring the distribution of the intensity of gamma radiation versus the energy of each photon. The study and analysis of gamma-ray spectra for scientific and technical use is called gamma spectroscopy, and gamma-ray spectrometers are the instruments which observe and collect such data. Because the energy of each photon of EM radiation is proportional to its frequency, gamma rays have sufficient energy that they are typically observed by counting individual photons.
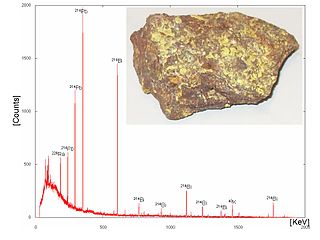
Gamma-ray spectroscopy is the qualitative study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acquire a quantitative spectrum measurement.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.

Neutron detection is the effective detection of neutrons entering a well-positioned detector. There are two key aspects to effective neutron detection: hardware and software. Detection hardware refers to the kind of neutron detector used and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.

Strontium aluminate is an aluminate compound with the chemical formula SrAl2O4. It is a pale yellow, monoclinic crystalline powder that is odourless and non-flammable. When activated with a suitable dopant, it acts as a photoluminescent phosphor with long persistence of phosphorescence.
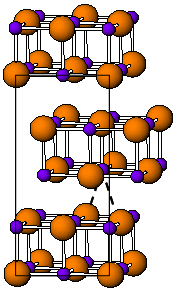
Thallium(I) iodide is a chemical compound with the formula TlI. It is unusual in being one of the few water-insoluble metal iodides, along with AgI, CuI, SnI2, SnI4, PbI2 and HgI2.

Magnesium iodide is an inorganic compound with the chemical formula MgI2. It forms various hydrates MgI2·xH2O. Magnesium iodide is a salt of magnesium and hydrogen iodide. These salts are typical ionic halides, being highly soluble in water.

Cerium(III) bromide is an inorganic compound with the formula CeBr3. This white hygroscopic solid is of interest as a component of scintillation counters.
Lanthanum(III) bromide (LaBr3) is an inorganic halide salt of lanthanum. When pure, it is a colorless white powder. The single crystals of LaBr3 are hexagonal crystals with melting point of 783 °C. It is highly hygroscopic and water-soluble. There are several hydrates, La3Br·x H2O, of the salt also known. It is often used as a source of lanthanum in chemical synthesis and as a scintillation material in certain applications.
In phosphors and scintillators, the activator is the element added as dopant to the crystal of the material to create desired type of nonhomogeneities.

A radionuclide identification device is a small, lightweight, portable gamma-ray spectrometer used for the detection and identification of radioactive substances. As RIIDs are portable, they are suitable for medical and industrial applications, fieldwork, geological surveys, first-line responders in Homeland Security, and Environmental Monitoring and Radiological Mapping along with other industries that necessitate the identification of radioactive substances..

Europium(II) iodide is the iodide salt of divalent europium cation.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.



















