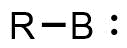In organic chemistry, a carbonyl group is a functional group with the formula C=O, composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.
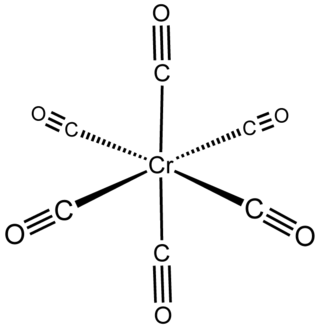
Chromium hexacarbonyl is a chromium(0) organometallic compound with the formula Cr(CO)6. It is a homoleptic complex, which means that all the ligands are identical. It is a colorless crystalline air-stable solid, with a high vapor pressure.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.
A carbon–oxygen bond is a polar covalent bond between atoms of carbon and oxygen. Carbon–oxygen bonds are found in many inorganic compounds such as carbon oxides and oxohalides, carbonates and metal carbonyls, and in organic compounds such as alcohols, ethers, carbonyl compounds and oxalates. Oxygen has 6 valence electrons of its own and tends to fill its outer shell with 8 electrons by sharing electrons with other atoms to form covalent bonds, accepting electrons to form an anion, or a combination of the two. In neutral compounds, an oxygen atom can form up to two single bonds or one double bond with carbon, while a carbon atom can form up to four single bonds or two double bonds with oxygen.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. They are analogous to metal nitrido complexes. Carbido complexes are a molecular subclass of carbides, which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis, and related catalytic industrial processes. Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Boronic acids and esters are classified depending on the type of carbon group (R) directly bonded to boron, for example alkyl-, alkenyl-, alkynyl-, and aryl-boronic esters. The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)2B-B(OR)2. For example, bis(pinacolato)diboron (B2Pin2), and bis(catecholato)diborane (B2Cat2) are common boron sources of this general formula.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.
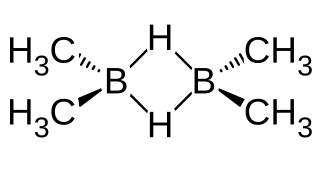
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
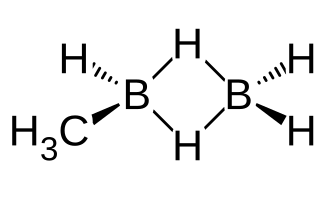
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3). Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.
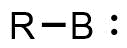
A borylene is the boron analogue of a carbene. The general structure is R-B: with R an organic moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping.
In organic chemistry, carboboration describes an addition of both a carbon and a boron moiety to certain carbon-containing double and triple bonds, such as alkenes, alkynes, and allenes.