Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name Piper, which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins.
A coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ions to ligands involves this kind of interaction. This type of interaction is central to Lewis acid–base theory.

A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, the lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis.

Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless, pyrophoric gas with a repulsively sweet odor. Synonyms include boroethane, boron hydride, and diboron hexahydride. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Its derivatives are useful reagents.
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory is a generalization of the Arrhenius theory.

The ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond, in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.

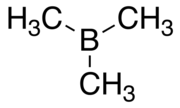
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
In chemistry, hydroboration refers to the addition of a hydrogen-boron bond to C-C, C-N, and C-O double bonds, as well as C-C triple bonds. This chemical reaction is useful in the organic synthesis of organic compounds. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the Nobel prize in chemistry with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates.
In chemistry a donor number (DN) is a quantitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 (antimony pentachloride), in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a zero DN. The units are kilocalories per mole for historical reasons. The donor number is a measure of the ability of a solvent to solvate cations and Lewis acids. The method was developed by V. Gutmann in 1976. Likewise Lewis acids are characterized by acceptor numbers (AN, see Gutmann–Beckett method).

An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can just be considered as a single product resulting from the direct combination of different molecules which comprises all the reactant molecules' atoms.

Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures wide over 200 °C, resistant to oxygen and water-tolerant.
In chemistry, a frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibit activation.

Carborane acidsH(CXB
11Y
5Z
6) (X, Y, Z = H, Alk, F, Cl, Br, CF3) are a class of superacids, some of which are estimated to be at least one million times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (H0 ≤ –18) and possess computed pKa values well below –20, establishing them as some of the strongest known Brønsted acids. The most well studied example is the highly chlorinated derivative H(CHB
11Cl
11). The acidity of H(CHB
11Cl
11) was found to vastly exceed that of triflic acid, CF
3SO
3H, and bistriflimide, (CF
3SO
2)
2NH, compounds previously regarded as the strongest isolable acids.
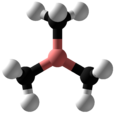
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.
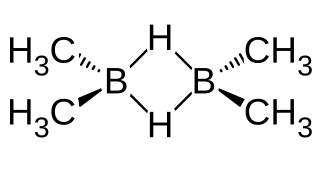
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
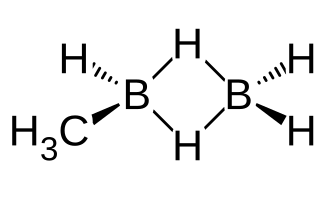
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3). Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.
In chemistry, the ECW model is a semi-quantitative model that describes and predicts the strength of Lewis acid–Lewis base interactions. Many chemical reactions can be described as acid–base reactions, so models for such interactions are of potentially broad interest. The model initially assigned E and C parameters to each and every acid and base. The model was later expanded to the ECW model to cover reactions that have a constant energy term, W, which describes processes that precede the acid–base reaction. This quantitative model is often discussed with the qualitative HSAB theory, which also seeks to rationalize the behavior of diverse acids and bases.

1,1-Dimethyldiborane is the organoboron compound with the formula (CH3)2B(μ-H)2BH2. A pair of related 1,2-dimethyldiboranes are also known. It is a colorless gas that ignites in air.