In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.

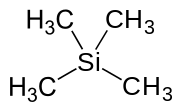
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.
Silicone grease, sometimes called dielectric grease, is a waterproof grease made by combining a silicone oil with a thickener. Most commonly, the silicone oil is polydimethylsiloxane (PDMS) and the thickener is amorphous fumed silica. Using this formulation, silicone grease is a translucent white viscous paste, with exact properties dependent on the type and proportion of the components. More specialized silicone greases are made from fluorinated silicones or, for low-temperature applications, PDMS containing some phenyl substituents in place of methyl groups. Other thickeners may be used, including stearates and powdered polytetrafluorethylene (PTFE). Greases formulated from silicone oils with silica thickener are sometimes referred to as silicone paste to distinguish them from silicone grease made with silicone oil and a soap thickener.
Carbon-13 (C13) nuclear magnetic resonance is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the 13
C
isotope. The main carbon isotope, 12
C
is not detected. Although much less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds.

Proton nuclear magnetic resonance is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H.
The heteronuclear single quantum coherence or heteronuclear single quantum correlation experiment, normally abbreviated as HSQC, is used frequently in NMR spectroscopy of organic molecules and is of particular significance in the field of protein NMR. The experiment was first described by Geoffrey Bodenhausen and D. J. Ruben in 1980. The resulting spectrum is two-dimensional (2D) with one axis for proton (1H) and the other for a heteronucleus, which is usually 13C or 15N. The spectrum contains a peak for each unique proton attached to the heteronucleus being considered. The 2D HSQC can also be combined with other experiments in higher-dimensional NMR experiments, such as NOESY-HSQC or TOCSY-HSQC.
Two-dimensional nuclear magnetic resonance spectroscopy is a set of nuclear magnetic resonance spectroscopy (NMR) methods which give data plotted in a space defined by two frequency axes rather than one. Types of 2D NMR include correlation spectroscopy (COSY), J-spectroscopy, exchange spectroscopy (EXSY), and nuclear Overhauser effect spectroscopy (NOESY). Two-dimensional NMR spectra provide more information about a molecule than one-dimensional NMR spectra and are especially useful in determining the structure of a molecule, particularly for molecules that are too complicated to work with using one-dimensional NMR.

Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.
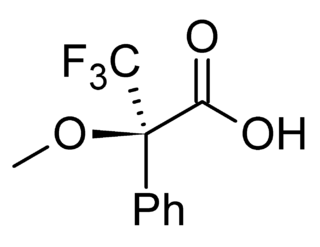
A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
In chemistry and molecular physics, fluxionalmolecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods.

Hexamethyldisiloxane (HMDSO or MM) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.

Fluorine-19 nuclear magnetic resonance spectroscopy is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy.

Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. It is colorless air sensitive liquid. It is a member of series (CH3)3-n(C6H5)2P that also includes n = 0, n = 2, and n = 3 that are often employed as ligands in metal phosphine complexes.
Nuclear magnetic resonance decoupling is a special method used in nuclear magnetic resonance (NMR) spectroscopy where a sample to be analyzed is irradiated at a certain frequency or frequency range to eliminate fully or partially the effect of coupling between certain nuclei. NMR coupling refers to the effect of nuclei on each other in atoms within a couple of bonds distance of each other in molecules. This effect causes NMR signals in a spectrum to be split into multiple peaks. Decoupling fully or partially eliminates splitting of the signal between the nuclei irradiated and other nuclei such as the nuclei being analyzed in a certain spectrum. NMR spectroscopy and sometimes decoupling can help determine structures of chemical compounds.
Nucleic acid NMR is the use of nuclear magnetic resonance spectroscopy to obtain information about the structure and dynamics of nucleic acid molecules, such as DNA or RNA. It is useful for molecules of up to 100 nucleotides, and as of 2003, nearly half of all known RNA structures had been determined by NMR spectroscopy.
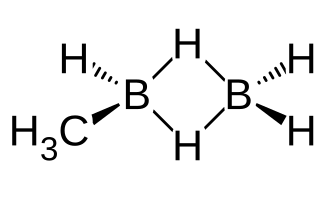
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3). Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.

Platinum-195 nuclear magnetic resonance spectroscopy is a spectroscopic technique which is used for the detection and characterisation of platinum compounds. The sensitivity of the technique and therefore its diagnostic utility have increased significantly starting from the 1970s, with 195Pt NMR nowadays considered the method of choice for structural elucidation of Pt species in solution.

1,3,5,7-Tetramethyl-1,3,5,7-tetrasilaadamantane is the organosilicon compound with the formula (CH2)6(SiCH3)4. It is a colorless solid that is soluble in organic solvents. The compound is one of the iconic carbosilanes, featuring alternating −Si−C−Si−C− linkages. Otherwise it can be described as a diamondoid cluster. It arises as one of many products from the pyrolysis of tetramethylsilane. A more efficient route involves the reaction of the cyclic carbosilane (CH2Si 2)3 (1,1,3,3,5,5-hexamethyl-1,3,5-trisilacyclohexane) with aluminium tribromide.