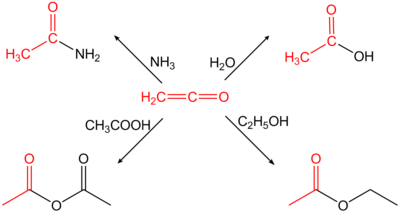
In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It is a tertiary amine, featuring a dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed acetate esters or simply acetates. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound.

Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether (monothioacetal-ester) in the presence of acetic anhydride.

Indene is an aromatic, polycyclic hydrocarbon with chemical formula C9H8. It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). In 2016 Schreiner and coworkers reported the first asymmetric variant of this reaction employing short oligopeptides as catalysts.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid described by the formula CH3CH=CHCO2H. The name crotonic acid was given because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. With a cis-alkene, Isocrotonic acid is an isomer of crotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to that of butyric acid.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
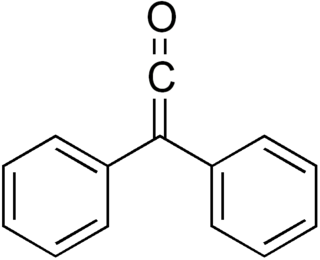
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
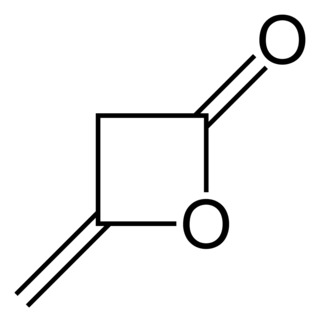
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
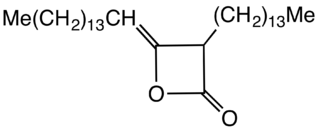
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.