Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structural formula H−C≡N. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature.

Zyklon B was the trade name of a cyanide-based pesticide invented in Germany in the early 1920s. It consists of hydrogen cyanide, as well as a cautionary eye irritant and one of several adsorbents such as diatomaceous earth. The product is notorious for its use by Nazi Germany during the Holocaust to murder approximately 1.1 million people in gas chambers installed at Auschwitz-Birkenau, Majdanek, and other extermination camps.
The Halabja massacre, also known as the Halabja chemical attack, was a massacre of Kurdish people that took place on 16 March 1988, during the Iraqi–Kurdish conflict in the closing days of the Iran–Iraq War in Halabja, Kurdistan, Iraq. The attack was part of the Al-Anfal Campaign in Kurdistan, as well as part of the Iraqi Army's attempt to repel the Iranian Operation Zafar 7. It took place 48 hours after the capture of the town by the Iranian Army. A United Nations (UN) medical investigation concluded that mustard gas was used in the attack, along with unidentified nerve agents.

Halabja is a city in the Kurdistan Region of Iraq and the capital of Halabja Governorate, located about 240 km (150 mi) northeast of Baghdad and 14 km (9 mi) from the Iranian border.
A blood agent is a toxic chemical agent that affects the body by being absorbed into the blood. Blood agents are fast-acting, potentially lethal poisons that typically manifest at room temperature as volatile colorless gases with a faint odor. They are either cyanide- or arsenic-based.

Tear gas, also known as a lachrymator agent or lachrymator, sometimes colloquially known as "mace" after the early commercial self-defense spray, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray, PAVA spray (nonivamide), CS gas, CR gas, CN gas, bromoacetone, xylyl bromide and Mace.
Xylyl bromide, also known as methylbenzyl bromide or T-stoff ('substance-T'), is any member or a mixture of organic chemical compounds with the molecular formula C6H4(CH3)(CH2Br). The mixture was formerly used as a tear gas and has an odor reminiscent of lilac. All members and the mixture are colourless liquids, although commercial or older samples appear yellowish.
Chemical, biological (CB) — and sometimes radiological — warfare agents were assigned what is termed a military symbol by the U.S. military until the American chemical and biological weapons programs were terminated. Military symbols applied to the CB agent fill, and not to the entire weapon. A chemical or biological weapon designation would be, for example, "Aero-14/B", which could be filled with GB, VX, TGB, or with a biological modification kit – OU, NU, UL, etc. A CB weapon is an integrated device of (1) agent, (2) dissemination means, and (3) delivery system.

Bromoacetone is an organic compound with the formula CH3COCH2Br. It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds.
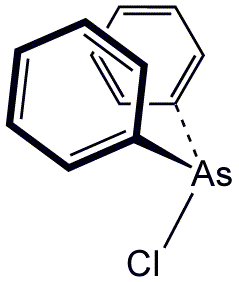
Diphenylchloroarsine (DA) is the organoarsenic compound with the formula (C6H5)2AsCl. It is highly toxic and was once used in chemical warfare. It is also an intermediate in the preparation of other organoarsenic compounds. The molecule consists of a pyramidal As(III) center attached to two phenyl rings and one chloride. It was also known as sneezing oil during World War I by the Allies.

syn-Propanethial S-oxide (or (Z)-propanethial S-oxide), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly "sulfines"), is a volatile liquid that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions, Allium cepa, as they are sliced. The release is due to the breaking open of the onion cells and their releasing enzymes called alliinases, which then break down amino acid sulfoxides, generating sulfenic acids. A specific sulfenic acid, 1-propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, called the lachrymatory factor synthase or LFS, giving syn-propanethial S-oxide. The gas diffuses through the air and, on contact with the eye, it stimulates sensory neurons creating a stinging, painful sensation. Tears are released from the tear glands to dilute and flush out the irritant. A structurally related lachrymatory compound, syn-butanethial S-oxide, C4H8OS, has been found in another genus Allium plant, Allium siculum.
White Cross (Weiẞkreuz) is a World War I chemical warfare agent consisting of one or more lachrymatory agents: bromoacetone (BA), bromobenzyl cyanide (Camite), bromomethyl ethyl ketone, chloroacetone, ethyl bromoacetate, and/or xylyl bromide.

Cyanide poisoning is poisoning that results from exposure to any of a number of forms of cyanide. Early symptoms include headache, dizziness, fast heart rate, shortness of breath, and vomiting. This phase may then be followed by seizures, slow heart rate, low blood pressure, loss of consciousness, and cardiac arrest. Onset of symptoms usually occurs within a few minutes. Some survivors have long-term neurological problems.

The Hypo helmet, or British Smoke Hood, was an early British World War I gas mask, designed by Cluny Macpherson

Ethyl iodoacetate is a chemical compound that is a derivative of ethyl acetate. Under normal conditions, the compound is a clear, light yellow to orange liquid.
The Black Veil Respirator was an early British gas mask designed by John Scott Haldane and introduced in May 1915.

Phenylcarbylamine chloride is a chemical compound that was used as a chemical warfare agent. It's an oily liquid with an onion-like odor. Classified as an isocyanide dichloride, this compound is a lung irritant with lachrymatory effects.

Cacodyl cyanide is a highly toxic organoarsenic compound discovered by Robert Bunsen in the 1840s. It is very volatile and flammable, as it shares the chemical properties of both arsenic and cyanide.

Tetrachlorodinitroethane is a chlorinated nitroalkane produced by nitration of tetrachloroethylene with dinitrogen tetroxide or fuming nitric acid. It's a powerful lachrymatory agent and pulmonary agent that is six times more toxic than chloropicrin. Tetrachlorodinitroethane may be used as a fumigant.

Bromomethyl ethyl ketone is a brominated ketone with lachrymatory effects. It was used as a chemical warfare agent in World War I. Bromomethyl ethyl ketone was developed as an alternative to bromoacetone, because acetone, the precursor to bromoacetone, was required for explosives production.













