
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O. The compound has few practical applications but is used in laboratories for research on new compounds of samarium.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.

Antimony trichloride is the chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as butter of antimony.

Gallium trichloride is the chemical compound with the formula GaCl3. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6. It is colourless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.
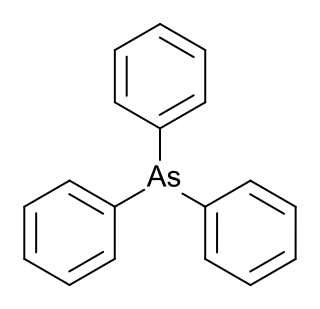
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.
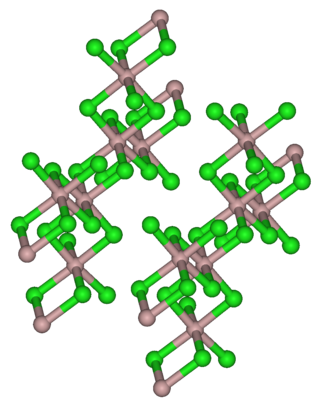
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions. It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.

Methyldichloroarsine, sometimes abbreviated "MD" and also known as methyl Dick, is an organoarsenic compound with the formula CH3AsCl2. This colourless volatile liquid is a highly toxic vesicant that has been used in chemical warfare.
Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.























