
Neptunium is a chemical element; it has symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has only one lattice constant, the distance between atoms, but in general lattices in three dimensions have six lattice constants: the lengths a, b, and c of the three cell edges meeting at a vertex, and the angles α, β, and γ between those edges.

Boron arsenide is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide B12As2. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).
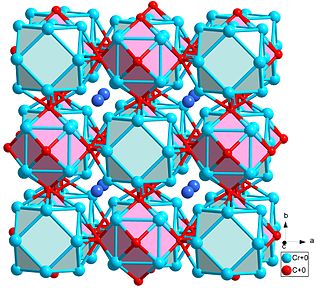
Cr23C6 is the prototypical compound of a common crystal structure, discovered in 1933 as part of the chromium-carbon binary phase diagram. Over 85 known compounds adopt this structure type, which can be described as a NaCl-like packing of chromium cubes and cuboctahedra.
Chromium hydrides are compounds of chromium and hydrogen, and possibly other elements. Intermetallic compounds with not-quite-stoichometric quantities of hydrogen exist, as well as highly reactive molecules. When present at low concentrations, hydrogen and certain other elements alloyed with chromium act as softening agents that enables the movement of dislocations that otherwise not occur in the crystal lattices of chromium atoms.
Plutonium selenide is a binary inorganic compound of plutonium and selenium with the chemical formula PuSe. The compound forms black crystals and does not dissolve in water.
Neptunium diarsenide is a binary inorganic compound of neptunium and arsenic with the chemical formula NpAs
2. The compound forms crystals.
Neptunium silicide is a binary inorganic compound of neptunium and silicon with the chemical formula NpSi
2. The compound forms crystals and does not dissolve in water.
Samarium(III) phosphide is an inorganic compound of samarium and phosphorus with the chemical formula SmP.

Terbium phosphide is an inorganic compound of terbium and phosphorus with the chemical formula TbP.
Plutonium phosphide is a binary inorganic compound of plutonium and phosphorus with the formula PuP.
Plutonium arsenide is a binary inorganic compound of plutonium and arsenic with the formula PuAs.
Samarium(III) arsenide is a binary inorganic compound of samarium and arsenic with the chemical formula SmAs.

Neodymium bismuthide or Bismuth-Neodymium is a binary inorganic compound of neodymium and bismuth with the formula NdBi. It forms crystals.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.
Ytterbium(II) sulfide is a binary inorganic compound of ytterbium and sulfur with the chemical formula YbS.

Praseodymium bismuthide is a binary inorganic compound of praseodymium and bismuth with the chemical formula of PrBi. It forms crystals.

Praseodymium antimonide is a binary inorganic compound of praseodymium and antimony with the formula PrSb.







