Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, persistent organic pollution in the environment.

Operation Ranch Hand was a U.S. military operation during the Vietnam War, lasting from 1962 until 1971. Largely inspired by the British use of chemicals 2,4,5-T and 2,4-D during the Malayan Emergency in the 1950s, it was part of the overall herbicidal warfare program during the war called "Operation Trail Dust". Ranch Hand involved spraying an estimated 19 million U.S. gallons (72,000 m3) of defoliants and herbicides over rural areas of South Vietnam in an attempt to deprive the Viet Cong of food and vegetation cover. Areas of Laos and Cambodia were also sprayed to a lesser extent. According to the Vietnamese government, the chemicals caused 400,000 deaths. The United States government has described these figures as unreliable.

The Seveso disaster was an industrial accident that occurred around 12:37 pm on 10 July 1976, in a small chemical manufacturing plant approximately 20 kilometres (12 mi) north of Milan in the Lombardy region of Italy. It resulted in the highest known exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in residential populations, which gave rise to numerous scientific studies and standardized industrial safety regulations, including the European Union's Seveso III Directive. This accident was ranked eighth in a list of the worst man-made environmental disasters by Time magazine in 2010.
Times Beach is a ghost town in St. Louis County, Missouri, United States, 17 miles (27 km) southwest of St. Louis and 2 miles (3 km) east of Eureka. Once home to more than two thousand people, the town was completely evacuated early in 1983 due to TCDD contamination, formerly the largest civilian exposure to the compound in the history of the United States.

Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.

A defoliant is any herbicidal chemical sprayed or dusted on plants to cause their leaves to fall off. Defoliants are widely used for the selective removal of weeds in managing croplands and lawns. Worldwide use of defoliants, along with the development of other herbicides and pesticides, allowed for the Green Revolution, an increase in agricultural production in mid-20th century. Defoliants have also been used in warfare as a means to deprive an enemy of food crops and/or hiding cover, most notably by the United Kingdom during the Malayan Emergency and the United States in the Vietnam War. Defoliants were also used by Indonesian forces in various internal security operations.

Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.

Hexachlorophene, also known as Nabac, is an organochlorine compound that was once widely used as a disinfectant. The compound occurs as a white odorless solid, although commercial samples can be off-white and possess a slightly phenolic odor. It is insoluble in water but dissolves in acetone, ethanol, diethyl ether, and chloroform. In medicine, hexachlorophene is useful as a topical anti-infective and anti-bacterial agent. It is also used in agriculture as a soil fungicide, plant bactericide, and acaricide.

Agent Green is the code name for a powerful herbicide and defoliant used by the U.S. military in its herbicidal warfare program during the Vietnam War. The name comes from the green stripe painted on the barrels to identify the contents. Largely inspired by the British use of herbicides and defoliants during the Malayan Emergency, it was one of the so-called "Rainbow Herbicides". Agent Green was only used between 1962 and 1964, during the early "testing" stages of the spraying program.

2,4,5-Trichlorophenoxyacetic acid, a synthetic auxin, is a chlorophenoxy acetic acid herbicide used to defoliate broad-leafed plants. It was developed in the late 1940s, synthesized by reaction of 2,4,5-Trichlorophenol and chloroacetic acid. It was widely used in the agricultural industry until being phased out, starting in the late 1970s due to toxicity concerns. Agent Orange, a defoliant used by the British in the Malayan Emergency and the U.S. in the Vietnam War, was equal parts 2,4,5-T and 2,4-D. 2,4,5-T itself is toxic with a NOAEL of 3 mg/kg/day and a LOAEL of 10 mg/kg/day. Agent Pink contained 100% 2,4,5-T. Additionally, the manufacturing process for 2,4,5-T contaminates this chemical with trace amounts of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD is a carcinogenic persistent organic pollutant with long-term effects on the environment. With proper temperature control during production of 2,4,5-T, TCDD levels can be held to about .005 ppm. Before the TCDD risk was well understood, early production facilities lacked proper temperature controls and individual batches tested later were found to have as much as 60 ppm of TCDD.

Agent Pink is the code name for a powerful herbicide and defoliant used by the U.S. military in its herbicidal warfare program during the Vietnam War. The name comes from the pink stripe painted on the barrels to identify the contents. Largely inspired by the British use of herbicides and defoliants during the Malayan Emergency, it was one of the rainbow herbicides that included the more infamous Agent Orange. Agent Pink was only used during the early "testing" stages of the spraying program before 1964.

Bis[2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl]oxalate is an organic compound with the formula (C5H11O2CC6HCl3O)2C2O2. A white solid, it is classified as a diester of oxalic acid. It is an active ingredient for the chemiluminescence in glowsticks. It can be synthesized by reacting 2-carbopentoxy-3,5,6-trichlorophenol with oxalyl chloride.

The Rainbow Herbicides are a group of tactical-use chemicals used by the United States military in Southeast Asia during the Vietnam War. Success with Project AGILE field tests in 1961 with herbicides in South Vietnam was inspired by the British use of herbicides and defoliants during the Malayan Emergency in the 1950s, which led to the formal herbicidal program Trail Dust. Herbicidal warfare is the use of substances primarily designed to destroy the plant-based ecosystem of an agricultural food production area and/or to destroy dense foliage which provides the enemy with natural tactical cover.
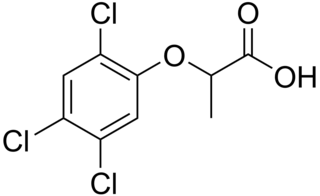
Fenoprop, also called 2,4,5-TP, is the organic compound 2-(2,4,5-trichlorophenoxy)propionic acid. It is a phenoxy herbicide and a plant growth regulator, an analog of 2,4,5-T in which the latter's acetic acid sidechain is replaced with a propionate group (with an extra CH3). The addition of this extra methyl group creates a chiral centre in the molecule and useful biological activity is found only in the (2R)-isomer. The compound's mechanism of action is to mimic the auxin growth hormone indoleacetic acid (IAA). When sprayed on plants it induces rapid, uncontrolled growth. As with 2,4,5-T, fenoprop is toxic to shrubs and trees.

2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic, defoliant, and glue preservative. It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.
A trichlorophenol is any organochloride of phenol that contains three covalently bonded chlorine atoms. Trichlorophenols are produced by electrophilic halogenation of phenol with chlorine. Different isomers of trichlorophenol exist according to which ring positions on the phenol contain chlorine atoms. 2,4,6-Trichlorophenol, for example, has two chlorine atoms in the ortho positions and one chlorine atom in the para position.

Coalite is a brand of low-temperature coke used as a smokeless fuel. The title refers to the residue left behind when coal is carbonised at 640 °C (1,184 °F). It was invented by Thomas Parker in 1904. In 1936 the Smoke Abatement Society awarded its inventor a posthumous gold medal.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.

Ohio River Park is a Superfund Site located in Neville Island, Pennsylvania. Between the 1920s-1970s, the Site was used for municipal waste, pesticide manufacturing, coke sludge disposal, cement manufacturing disposal, and pesticide waste. In 1977, Neville Land Company donated the Site to Allegheny County who started developing the Site as a community park. In 1979, Allegheny County found various hazardous contaminants on the Site. On August 30, 1990, the Site was determined to be a Superfund Site due to VOCs, SVOCs, inorganics, and pesticides being present in the surface soil, subsurface soil, surface water, river sediment, and groundwater. Soil remediation began in February 1998 and ended in September 1999. Today, Ohio River Park has the Robert Morris University Island Sports Center and Coraopolis Bridge on top of it. Additionally, benzene continues to be monitored because it is still present in the Site's groundwater.
Janet Mary McVeagh was a New Zealand disability worker, environmentalist and politician who was a co-leader of the Values Party in the 1980s.
















