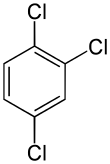
1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Nonane is a linear alkane hydrocarbon with the chemical formula C9H20. It is a colorless, flammable liquid, occurring primarily in the component of the petroleum distillate fraction commonly called kerosene, which is used as a heating, tractor, and jet fuel. Nonane is also used as a solvent, distillation chaser, fuel additive, and a component in biodegradable detergents.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index, very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is soluble in about 800 parts water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
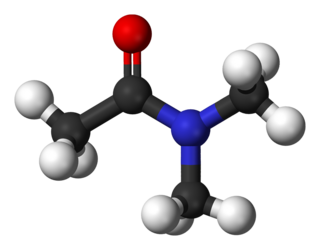
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.

Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals, primarily phenol and acetone.
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.

1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
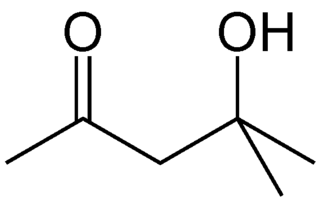
Diacetone alcohol is an organic compound with the formula CH3C(O)CH2C(OH)(CH3)2, sometimes called DAA. This colorless liquid is a common synthetic intermediate used for the preparation of other compounds, and is also used as a solvent.

Hexachlorobutadiene, (often abbreviated as "HCBD") Cl2C=C(Cl)C(Cl)=CCl2, is a colorless liquid at room temperature that has an odor similar to that of turpentine. It is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds.

Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.

4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including antioxidants commonly found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.
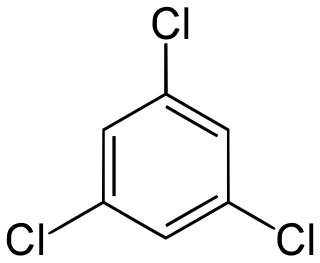
1,3,5-Trichlorobenzene is an organochlorine compound. It is one of the three isomers of trichlorobenzene. Being more symmetrical than the other isomers, it exists as colourless crystals whereas the other isomers are liquids at room temperature.
Bis(chloroethyl) ether is an organic compound with the formula O(CH2CH2Cl)2. It is an ether with two 2-chloroethyl substituents. It is a colorless liquid with the odor of a chlorinated solvent.

Perchloromethyl mercaptan is the organosulfur compound with the formula CCl3SCl. It is mainly used as an intermediate for the synthesis of dyes and fungicides (captan, folpet). It is a colorless oil, although commercial samples are yellowish. It is insoluble in water but soluble in organic solvents. It has a foul, unbearable, acrid odor. Perchloromethyl mercaptan is the original name. The systematic name is trichloromethanesulfenyl chloride, because the compound is a sulfenyl chloride, not a mercaptan.
1,2,3-Trichlorobenzene is a organochlorine compound with the chemical formula C6H3Cl3. This is one of three isomers of trichlorobenzene with two others being 1,2,4-Trichlorobenzene and 1,3,5-Trichlorobenzene.