In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
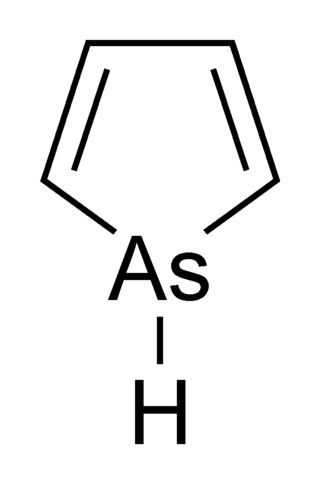
Arsole, also called arsenole or arsacyclopentadiene, is an organoarsenic compound with the formula C4H4AsH. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole. Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.

TATB, triaminotrinitrobenzene or 2,4,6-triamino-1,3,5-trinitrobenzene is an aromatic explosive, based on the basic six-carbon benzene ring structure with three nitro functional groups (NO2) and three amine (NH2) groups attached, alternating around the ring.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.

1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its detonation velocity is 7,350 meters per second, which is comparable to TATB (triaminotrinitrobenzene).
The Blanc chloromethylation is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923

Trimesic acid, also known as benzene-1,3,5-tricarboxylic acid, is an organic compound with the formula C6H3(CO2H)3. It is one of three isomers of benzenetricarboxylic acid. A colorless solid, trimesic acid has some commercial value as a precursor to some plasticizers.
1,2,4-Trichlorobenzene is an organochlorine compound, one of three isomers of trichlorobenzene. It is a derivative of benzene with three chloride substituents. It is a colorless liquid used as a solvent for a variety of compounds and materials.

Trichlorobenzene (TCB) may refer to any of three isomeric chlorinated derivatives of benzene with the molecular formula C6H3Cl3. Trichlorobenzenes are man-made chemical compounds that occur in three different forms. Even though the forms have the same molecular weight and molecular formulae, they are structurally different by the positions of the chlorine atoms attached to the benzene ring. 1,2,3-Trichlorobenzene and 1,3,5-trichlorobenzene are colorless solids, but 1,2,4-trichlorobenzene is a colorless oil. The isomers may also have different chemical and toxicological properties.

Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.

1,3,5-Trinitrobenzene is an organic compound with the formula C6H3(NO2)3. It is one of three trinitrated benzene-derivatives. A pale yellow solid, the compound is highly explosive.

The trihydroxybenzenes (or benzenetriols) are organic compounds with the formula C6H3(OH)3. Also classified as polyphenols, they feature three hydroxyl groups substituted onto a benzene ring. They are white solids with modest solubility in water.
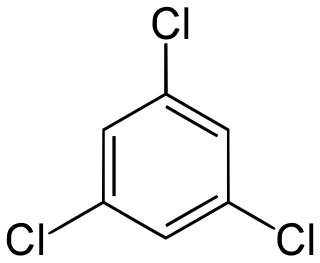
1,3,5-Trichlorobenzene is an organochlorine compound. It is one of the three isomers of trichlorobenzene. Being more symmetrical than the other isomers, it exists as colourless crystals whereas the other isomers are liquids at room temperature.

The C3-benzenes are a class of organic aromatic compounds which contain a benzene ring and three other carbon atoms. For the hydrocarbons with no further unsaturation, there are four isomers. The chemical formula for all the saturated isomers is C9H12.

1,3,5-Triethylbenzene is a chemical compound of the group of aromatic hydrocarbons.
Tetrachlorobenzene is any of three isomeric chlorinated derivatives with the molecular formula C6H2Cl4. They differ by the positions of the chlorine atoms around the ring. Tetrachlorobenzenes are colorless crystalline compounds.
















