Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by a large steric hindrance from the substitutions. The first isolated diphosphene bis(2,4,6-tri-tert-butylphenyl)diphosphene was exemplified by Masaaki Yoshifuji and his coworkers in 1981, in which diphosphene is stabilized by two bulky phenyl group.
Clemmensen reduction is a chemical reaction described as a reduction of ketones or aldehydes to alkanes using zinc amalgam and concentrated hydrochloric acid (HCl). This reaction is named after Erik Christian Clemmensen, a Danish-American chemist.
The Corey–House synthesis is an organic reaction that involves the reaction of a lithium diorganylcuprate with an organic halide or pseudohalide to form a new alkane, as well as an ill-defined organocopper species and lithium (pseudo)halide as byproducts.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.
A carbometallation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometallation.

Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, referencing Kazuhiko Takai, who first reported it in 1986. In the original reaction, the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride and the product is a vinyl halide. One main advantage of this reaction is the E-configuration of the double bond that is formed. According to the original report, existing alternatives such as the Wittig reaction only gave mixtures.
Chiral Lewis acids (CLAs) are a type of Lewis acid catalyst. These acids affect the chirality of the substrate as they react with it. In such reactions, synthesis favors the formation of a specific enantiomer or diastereomer. The method is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically a metal, such as indium, zinc, lithium, aluminium, titanium, or boron. The chiral-altering ligands employed for synthesizing these acids often have multiple Lewis basic sites that allow the formation of a ring structure involving the metal atom.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
Marjorie Constance Caserio was an English chemist. In 1975, she was awarded the Garvan Medal by the American Chemical Society.

Copper hydride is inorganic compound with the chemical formula CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.
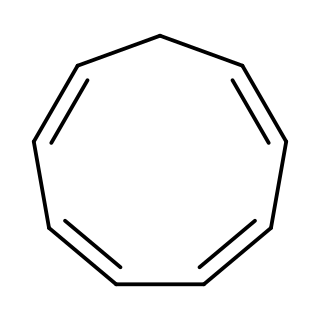
Cyclononatetraene is an organic compound with the formula C9H10. It was first prepared in 1969 by protonation of the corresponding aromatic anion (described below). It is unstable and isomerizes with a half-life of 50 minutes at room temperature to 3a,7a-dihydro-1H-indene via a thermal 6π disrotatory electrocyclic ring closing. Upon exposure to ultraviolet light, it undergoes a photochemical 8π electrocyclic ring closing to give bicyclo[6.1.0]nona-2,4,6-triene.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.
Germanium(II) hydrides, also called germylene hydrides, are a class of Group 14 compounds consisting of low-valent germanium and a terminal hydride. They are also typically stabilized by an electron donor-acceptor interaction between the germanium atom and a large, bulky ligand.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
Manganese(III) chloride is the hypothetical inorganic compound with the formula MnCl3.
Ruthenium(II) chloride is an inorganic compound, a metal salt of ruthenium and hydrochloric acid with the formula RuCl
2.
Bromocyclopropane is a organobromine compound with the chemical formula C3H5Br. It is a member of haloalkane family.

Jiro Tsuji was a Japanese chemist, notable for his discovery of organometallic reactions, including the Tsuji-Trost reaction, the Tsuji-Wilkinson decarbonylation, and the Tsuji-Wacker reaction.