
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (−OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, persistent organic pollution in the environment.

The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944.
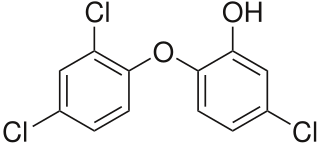
Triclosan is an antibacterial and antifungal agent present in some consumer products, including toothpaste, soaps, detergents, toys, and surgical cleaning treatments. It is similar in its uses and mechanism of action to triclocarban. Its efficacy as an antimicrobial agent, the risk of antimicrobial resistance, and its possible role in disrupted hormonal development remains controversial. Additional research seeks to understand its potential effects on organisms and environmental health.

Parabens are chemicals that are commonly used as preservatives in cosmetic and pharmaceutical products. Chemically, they are a series of parahydroxybenzoates or esters of parahydroxybenzoic acid. Research is being conducted to evaluate the potential health implications of paraben usage.

Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843.
A chlorophenol is any organochloride of phenol that contains one or more covalently bonded chlorine atoms. There are five basic types of chlorophenols and 19 different chlorophenols in total when positional isomerism is taken into account. Chlorophenols are produced by electrophilic halogenation of phenol with chlorine.

2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic, defoliant, and glue preservative. It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.
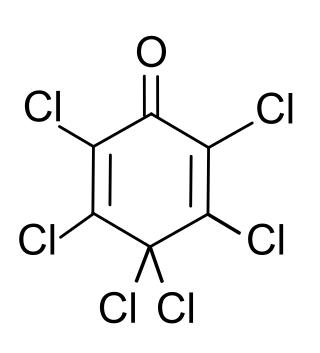
Hexachlorocyclohexa-2,5-dien-1-one, sometimes informally called hexachlorophenol (HCP) is an organochlorine compound. It can be prepared from phenol. Despite the informal name, the compound is not a phenol but is a ketone. The informal name is derived from its method of preparation which includes phenol as a reagent.
Dichlorophenols (DCPs) are any of several chemical compounds which are derivatives of phenol containing two chlorine atoms. There are six isomers:
2,6-Dichlorophenol is a compound with formula C6H3Cl2OH. It is one of the six isomers of dichlorophenol. It is a colorless solid. Its pKa is 6.78, which is about 100x more acidic than 2-chlorophenol (8.52) and 1000x more acidic than phenol itself (9.95).

Polychloro phenoxy phenols are a group of organic polyhalogenated compounds. Among them include triclosan and predioxin which can degrade to produce certains types of dioxins and furans. Notably, however, the particular dioxin formed by degradation of triclosan, 2,8-DCDD, was found to be non-toxic in fish embryos.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin (sometimes shortened, though inaccurately, to simply 'dioxin') with the chemical formula C12H4Cl4O2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.

3-Chlorophenol is an organic compound with the molecular formula C6H4ClOH. It is one of three isomers of monochlorophenol. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Together with 3,5-dichlorophenol, it is prepared industrially by dechlorination of polychlorophenols. Alternatively, it arises via the cumene process, which starts with the alkylation of chlorobenzene with propylene.
Adsorbable organic halides (AOX) is a measure of the organic halogen load at a sampling site such as soil from a land fill, water, or sewage waste. The procedure measures chlorine, bromine, and iodine as equivalent halogens, but does not measure fluorine levels in the sample.
4-Chlorophenol is an organic compound with the formula C6H4ClOH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.



















