Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison.

Sarin is an extremely toxic synthetic organophosphorus compound. A colourless, odourless liquid, it is used as a chemical weapon due to its extreme potency as a nerve agent. Exposure is lethal even at very low concentrations, where death can occur within one to ten minutes after direct inhalation of a lethal dose, due to suffocation from respiratory paralysis, unless antidotes are quickly administered. People who absorb a non-lethal dose and do not receive immediate medical treatment may suffer permanent neurological damage.

Soman is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).

In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the binding site, and residues that catalyse a reaction of that substrate, the catalytic site. Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes.

VX is an extremely toxic synthetic chemical compound in the organophosphorus class, specifically, a thiophosphonate. In the class of nerve agents, it was developed for military use in chemical warfare after translation of earlier discoveries of organophosphate toxicity in pesticide research. In recent years, VX was found to be the agent used in the assassination of Kim Jong-nam. In its pure form, VX is an oily, relatively non-volatile liquid that is amber-like in colour. Because of its low volatility, VX persists in environments where it is dispersed.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.

Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinesterase. It is commonly available over the counter as a nutrient supplement, and is marketed as a cognitive enhancer for improving memory and concentration.

AEBSF or 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride is a water-soluble, irreversible serine protease inhibitor with a molecular weight of 239.5 Da. It inhibits proteases like chymotrypsin, kallikrein, plasmin, thrombin, and trypsin. The specificity is similar to the inhibitor PMSF, nevertheless AEBSF is more stable at low pH values. Typical usage is 0.1 - 1.0 mM. AEBSF was first reported for use in biochemistry in 1993, and came into common use for the inhibition serine proteases and of non-protease enzymes such as acetylhydrolases in the mid 1990s.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

In biochemistry, phenylmethylsulfonyl fluoride (PMSF) is a serine protease inhibitor commonly used in the preparation of cell lysates. PMSF does not inactivate all serine proteases. The effective concentration of PMSF is between 0.1 - 1 mM. The half-life is short in aqueous solutions. At 4˚C, pH 8, PMSF is almost completely degraded after 1 day. Stock solutions are usually made up in anhydrous ethanol, isopropanol, or corn oil and diluted immediately before use.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

In molecular biology, Proteinase K is a broad-spectrum serine protease. The enzyme was discovered in 1974 in extracts of the fungus Parengyodontium album. Proteinase K is able to digest hair (keratin), hence, the name "Proteinase K". The predominant site of cleavage is the peptide bond adjacent to the carboxyl group of aliphatic and aromatic amino acids with blocked alpha amino groups. It is commonly used for its broad specificity. This enzyme belongs to Peptidase family S8 (subtilisin). The molecular weight of Proteinase K is 28,900 daltons.

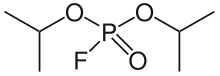
The enzyme diisopropyl-fluorophosphatase (EC 3.1.8.2) catalyzes the reaction

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.

Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.

IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.

Guanitoxin (GNT), formerly known as anatoxin-a(S) "Salivary", is a naturally occurring cyanotoxin commonly isolated from cyanobacteria. It is a potent covalent acetylcholinesterase inhibitor, and thus a potent rapid acting neurotoxin which in cases of severe exposure can lead to death. Guanitoxin was first structurally characterized in 1989, and consists of a cyclic N-hydroxyguanine organophosphate with a phosphate ester moiety.