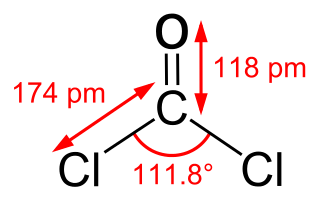
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

In organic chemistry, isocyanate is the functional group with the formula R−N=C=O. Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers.

Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane and methyl carbylamine. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As an extremely toxic and irritating compound, it is very hazardous to human health. It was the principal toxicant involved in the infamous Bhopal gas disaster, which officially killed around 20,000 people in total. It is also a very potent lachrymatory agent.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Oxazolidine is a five-membered heterocycle ringwith the formula (CH2)3(NH)O.The O atom and NH groups are not mutually bonded, in contrast to isoxazolidine. Oxazolidines are derivatives of the parent oxazolidine owing to the presence of substituents on carbon and/or nitrogen. Oxazolines are unsaturated analogues of oxazolidines.
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively.

Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.

Methylene diphenyl diisocyanate (MDI) is an aromatic diisocyanate. Three isomers are common, varying by the positions of the isocyanate groups around the rings: 2,2′-MDI, 2,4′-MDI, and 4,4′-MDI. The 4,4′ isomer is most widely used, and is also known as 4,4′-diphenylmethane diisocyanate. This isomer is also known as Pure MDI. MDI reacts with polyols in the manufacture of polyurethane. It is the most produced diisocyanate, accounting for 61.3% of the global market in the year 2000.
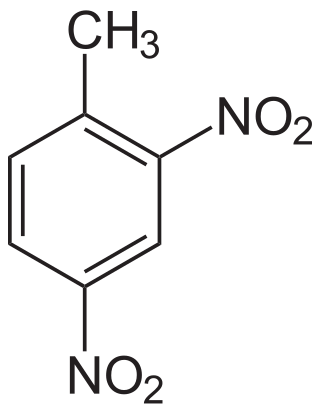
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.

Polyisocyanurate, also referred to as PIR, polyiso, or ISO, is a thermoset plastic typically produced as a foam and used as rigid thermal insulation. The starting materials are similar to those used in polyurethane (PUR) except that the proportion of methylene diphenyl diisocyanate (MDI) is higher and a polyester-derived polyol is used in the reaction instead of a polyether polyol. The resulting chemical structure is significantly different, with the isocyanate groups on the MDI trimerising to form isocyanurate groups which the polyols link together, giving a complex polymeric structure.

Isophorone diisocyanate (IPDI) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is produced in relatively small quantities, accounting for only 3.4% of the global diisocyanate market in the year 2000. Aliphatic diisocyanates are used, not in the production of polyurethane foam, but in special applications, such as enamel coatings which are resistant to abrasion and degradation from ultraviolet light. These properties are particularly desirable in, for instance, the exterior paint applied to aircraft.
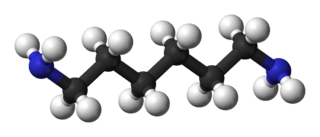
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.
Moisture-cure polyurethanes -- or polyurethane prepolymer -- are isocyanate-terminated prepolymers that are formulated to cure with ambient water. Cured PURs are segmented copolymer polyurethane-ureas exhibiting microphase-separated morphologies. One phase is derived from a typically flexible polyol that is generally referred to as the “soft phase”. Likewise the corresponding “hard phase” is born from the di- or polyisocyanates that through water reaction produce a highly crosslinked material with softening temperature well above room temperature.
Polyurethane dispersion, or PUD, is understood to be a polyurethane polymer resin dispersed in water, rather than a solvent, although some cosolvent maybe used. Its manufacture involves the synthesis of polyurethanes having carboxylic acid functionality or nonionic hydrophiles like PEG incorporated into, or pendant from, the polymer backbone. Two component polyurethane dispersions are also available.

Tetramethylxylylene diisocyanate (TMXDI) is an organic compound with the formula C6H4(CMe2NCO)2 (Me = CH3). Introduced in the 1980s by American Cyanamid, the molecule features two isocyanate groups. TMXDI is generally classified as an aliphatic isocyanate, which are generally more UV stable than their aromatic counterparts.

UV-328 is a chemical compound that belongs to the phenolic benzotriazoles. It is a UV filter that is used as an antioxidant for plastics.

Dimethylolpropionic acid (DMPA) is a chemical compound that has the full IUPAC name of 2,2-bis(hydroxymethyl)propionic acid and is an organic compound with one carboxyl and two hydroxy groups. It has the CAS Registry Number of 4767-03-7.
Hydrogenated MDI (H12MDI or 4,4′-diisocyanato dicyclohexylmethane) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is a water white liquid at room temperature and is manufactured in relatively small quantities. It is also known as 4,4'-methylenedi(cyclohexyl isocyanate) or methylene bis(4-cyclohexylisocyanate) and has the formula CH2[(C6H10)NCO]2.
Blocked isocyanates are organic compounds that have their isocyanate functionality chemically blocked to control reactivity. They are the product of an isocyanate moiety and a suitable blocking agent. It may also be a polyurethane prepolymer that is NCO terminated but this functionality has also been chemically reacted with a blocking agent. They are usually used in polyurethane applications but not always. They are extensively used in industrial applications such as coatings, sealants and adhesives.
















