Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison.

Mustard gas or sulfur mustard is any of several chemical compounds that contain the chemical structure S(CH2CH2Cl)2. In the wider sense, compounds with the substituent S(CH2CH2X)2 and N(CH2CH2X)3 are known as sulfur mustards and nitrogen mustards, respectively, where X = Cl or Br. Such compounds are potent alkylating agents, which can interfere with several biological processes. Also known as mustard agents, this family of compounds are infamous cytotoxins and blister agents with a long history of use as chemical weapons. The name mustard gas is technically incorrect: the substances, when dispersed, are often not gases but a fine mist of liquid droplets. Sulfur mustards are viscous liquids at room temperature and have an odor resembling mustard plants, garlic, or horseradish, hence the name. When pure, they are colorless, but when used in impure forms, such as in warfare, they are usually yellow-brown. Mustard gases form blisters on exposed skin and in the lungs, often resulting in prolonged illness ending in death. The typical mustard gas is the organosulfur compound bis(2-chloroethyl) sulfide.

Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare, biological warfare and radiological warfare, which together make up CBRN, the military acronym for chemical, biological, radiological, and nuclear, all of which are considered "weapons of mass destruction" (WMDs), a term that contrasts with conventional weapons.

The use of toxic chemicals as weapons dates back thousands of years, but the first large-scale use of chemical weapons was during World War I. They were primarily used to demoralize, injure, and kill entrenched defenders, against whom the indiscriminate and generally very slow-moving or static nature of gas clouds would be most effective. The types of weapons employed ranged from disabling chemicals, such as tear gas, to lethal agents like phosgene, chlorine, and mustard gas. This chemical warfare was a major component of the first global war and first total war of the 20th century. The killing capacity of gas was limited, with about 90,000 fatalities from a total of 1.3 million casualties caused by gas attacks. Gas was unlike most other weapons of the period because it was possible to develop countermeasures, such as gas masks. In the later stages of the war, as the use of gas increased, its overall effectiveness diminished. The widespread use of these agents of chemical warfare, and wartime advances in the composition of high explosives, gave rise to an occasionally expressed view of World War I as "the chemist's war" and also the era where weapons of mass destruction were created.

Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a chemical weapon, acting as a vesicant and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to geraniums.

A blister agent, is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as cantharidin are also blister-producing agents (vesicants). Furanocoumarin, another naturally occurring substance, causes vesicant-like effects indirectly, for example, by increasing skin photosensitivity greatly. Vesicants have medical uses including wart removal but can be dangerous if even small amounts are ingested.

A chemical burn occurs when living tissue is exposed to a corrosive substance or a cytotoxic agent. Chemical burns follow standard burn classification and may cause extensive tissue damage. The main types of irritant and/or corrosive products are: acids, bases, oxidizers / reducing agents, solvents, and alkylants. Additionally, chemical burns can be caused by biological toxins and by some types of cytotoxic chemical weapons, e.g., vesicants such as mustard gas and Lewisite, or urticants such as phosgene oxime.

Chlormethine, also known as mechlorethamine, mustine, HN2, and embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Xylyl-bromide, also known as methylbenzyl bromide or T-stoff ('substance-T'), is any member or a mixture of organic chemical compounds with the molecular formula C6H4(CH3)(CH2Br). The mixture was formerly used as a tear gas and has an odor reminiscent of lilac. All members and the mixture are colourless liquids, although commercial or older samples appear yellowish.
Chemical, biological (CB) — and sometimes radiological — warfare agents were assigned what is termed a military symbol by the U.S. military until the American chemical and biological weapons programs were terminated. Military symbols applied to the CB agent fill, and not to the entire weapon. A chemical or biological weapon designation would be, for example, "Aero-14/B", which could be filled with GB, VX, TGB, or with a biological modification kit – OU, NU, UL, etc. A CB weapon is an integrated device of (1) agent, (2) dissemination means, and (3) delivery system.

2-Chloro-N,N-bis(2-chloroethyl)ethanamine, also known as trichlormethine, tris(2-chloroethyl)amine is the organic compound with the formula N(CH2CH2Cl)3. Often abbreviated HN3 or HN-3, it is a powerful blister agent and a nitrogen mustard used for chemical warfare. HN3 was the last of the nitrogen mustard agents developed. It was designed as a military agent and is the only one of the nitrogen mustards that is still used for military purposes. It is the principal representative of the nitrogen mustards because its vesicant properties are almost equal to those of HD and thus the analogy between the two types of mustard is the strongest. As a vesicant the use and production is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.

Sesquimustard is the organosulfur compound with the formula (ClCH2CH2SCH2)2. Although it is a colorless solid, impure samples are often brown. The compound is a type of mustard gas, a vesicant used as a chemical weapon. From the chemical perspective, the compound is both a thioether and an alkyl chloride.

SS John Harvey was a U.S. World War II Liberty ship. This ship is best known for carrying a secret cargo of mustard gas and whose sinking by German aircraft in December 1943 at the port of Bari in south Italy caused an unintentional release of chemical weapons.
Yellow Cross (Gelbkreuz) is a World War I chemical warfare agent usually based on sulfur mustard.
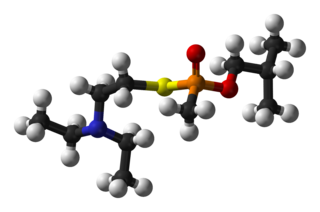
VR is a "V-series" unitary nerve agent closely related to the better-known VX nerve agent. It became a prototype for the series of Novichok agents. According to chemical weapons expert Jonathan Tucker, the first binary formulation developed under the Soviet Foliant program was used to make Substance 33, differing from VX only in the alkyl substituents on its nitrogen and oxygen atoms. "This weapon was given the code name Novichok."
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as a weapon "or its precursor that can cause death, injury, temporary incapacitation or sensory irritation through its chemical action. Munitions or other delivery devices designed to deliver chemical weapons, whether filled or unfilled, are also considered weapons themselves."
Chemical weapons have been a part of warfare in most societies for centuries. However, their usage has been extremely controversial since the 20th century.

Bis(2-chloroethyl)sulfide is the organosulfur compound with the formula (ClCH2CH2)2S. It is a prominent member of a family of cytotoxic and blister agents known as mustard agents. Sometimes referred to as mustard gas, the term is technically incorrect: bis(2-chloroethyl)sulfide is a liquid at room temperature. In warfare it was dispersed in the form of a fine mist of liquid droplets.










