
Phenytoin (PHT), sold under the brand name Dilantin among others, is an anti-seizure medication. It is useful for the prevention of tonic-clonic seizures and focal seizures, but not absence seizures. The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines. It may also be used for certain heart arrhythmias or neuropathic pain. It can be taken intravenously or by mouth. The intravenous form generally begins working within 30 minutes and is effective for roughly 24 hours. Blood levels can be measured to determine the proper dose.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

Tramadol, sold under the brand name Ultram among others, is an opioid pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).
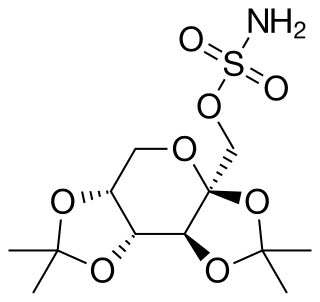
Topiramate, sold under the brand name Topamax among others, is a medication used to treat epilepsy and prevent migraines. It has also been used in alcohol dependence and essential tremor. For epilepsy this includes treatment for generalized or focal seizures. It is taken orally.

Lamotrigine, sold under the brand name Lamictal among others, is a medication used to treat epilepsy and stabilize mood in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome. In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression for all groups except in the severely depressed; but for patients with bipolar disorder who are not currently symptomatic, it appears to reduce the risk of future episodes of depression.

Oxcarbazepine, sold under the brand name Trileptal among others, is a medication used to treat epilepsy. For epilepsy it is used for both focal seizures and generalized seizures. It has been used both alone and as add-on therapy in people with bipolar disorder who have had no success with other treatments. It is taken by mouth.
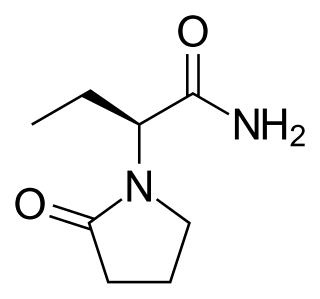
Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy. It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.

Clonazepam, sold under the brand names Klonopin, Rivotril and Paxam, is a medication used to prevent and treat anxiety disorders, seizures, bipolar mania, agitation associated with psychosis, OCD and akathisia. It is a long-acting tranquilizer of the benzodiazepine class. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. It is typically taken by mouth but is also used intravenously. Effects begin within one hour and last between eight and twelve hours in adults.
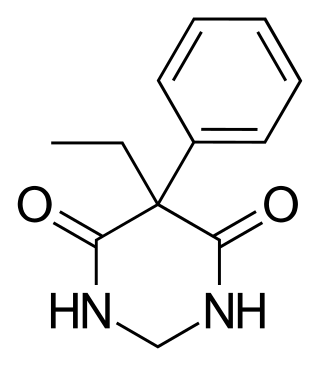
Primidone, sold under various brand names, is a barbiturate medication that is used to treat partial and generalized seizures and essential tremors. It is taken by mouth.

Vigabatrin, sold under the brand names Sabril and Vigpoder, is a medication used to treat epilepsy. It became available as a generic medication in 2019.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.
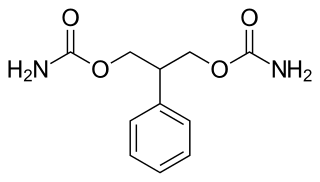
Felbamate is an anticonvulsant used in the treatment of epilepsy. It is used to treat partial seizures in adults and partial and generalized seizures associated with Lennox–Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drug's usage to severe refractory epilepsy.

Brivaracetam, sold under the brand name Briviact among others, is a chemical analog of levetiracetam, a racetam derivative with anticonvulsant (antiepileptic) properties. It is marketed by the pharmaceutical company UCB.

Lacosamide, sold under the brand name Vimpat among others, is a medication used for the treatment of partial-onset seizures and primary generalized tonic-clonic seizures. It is used by mouth or intravenously.
Drug rash with eosinophilia and systemic symptoms or drug reaction with eosinophilia and systemic symptoms (DRESS), also termed drug-induced hypersensitivity syndrome (DIHS), is a rare reaction to certain medications. It involves primarily a widespread skin rash, fever, swollen lymph nodes, and characteristic blood abnormalities such as an abnormally high level of eosinophils, low number of platelets, and increased number of atypical white blood cells (lymphocytes). However, DRESS is often complicated by potentially life-threatening inflammation of internal organs and the syndrome has about a 10% mortality rate. Treatment consists of stopping the offending medication and providing supportive care. Systemic corticosteroids are commonly used as well but no controlled clinical trials have assessed the efficacy of this treatment.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.

Perampanel, sold under the brand name Fycompa, is an anti-epileptic medication developed by Eisai Co. that is used in addition to other drugs to treat partial seizures and generalized tonic-clonic seizures for people older than twelve years. It was first approved in 2012, and as of 2016, its optimal role in the treatment of epilepsy relative to other drugs was not clear. It was the first antiepileptic drug in the class of selective non-competitive antagonist of AMPA receptors.

Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is an anticonvulsant medication approved for use in Europe and the United States as monotherapy or as additional therapy for partial-onset seizures epilepsy.
An analgesic adjuvant is a medication that is typically used for indications other than pain control but provides control of pain (analgesia) in some painful diseases. This is often part of multimodal analgesia, where one of the intentions is to minimize the need for opioids.
Antimanic drugs are psychotropic drugs that are used to treat symptoms of mania. Though there are different causes of mania, the majority is caused by bipolar disorder, therefore antimanic drugs are mostly similar to drugs treating bipolar disorder. Since 1970s, antimanic drugs have been used specifically to control the abnormal elevation of mood or mood swings during manic episodes. One purpose of antimanic drugs is to alleviate or shorten the duration of an acute mania. Another objective is to prevent further cycles of mania and maintain the improvement achieved during the acute episode. The mechanism of antimanic drugs has not yet been fully known, it is proposed that they mostly affect chemical neurotransmitters in the brain. However, the usage of antimanic drugs should be consulted with a doctor or pharmacist due to their side effects and interactions with other drugs and food.



















